Unbelievable Sulphuric Acid And Ammonia Balanced Equation

Give your answer to 2 significant figures.
Sulphuric acid and ammonia balanced equation. 3 The sulphuric acid catalyst is added to speed up the reaction. This is often by reacting the sulfuric acid with ammonia or ammonium hydroxide solution to make ammonium sulfate NH42SO4. Ammonia sulfuric acid ammonium sulfate ammonium.
Na2SO3 H2SO4 ---- Na2SO4 H2O SO2 1 Above 200 0 C the nitric acid decomposes into H 2 O NO 2 and O 2 as it is very unstable to heat. What happens when ammonia reacts with Sulphuric acidWhat is the chemical formula for ammonium sulphate. Calculate the pH of a 0280moldm solution of sulphuric acid.
Chemistry Chemical Reactions Chemical Reactions and Equations. What Volume Of 0250M Sulfuric Acid Solution Would Be Needed To React Complately With 1800 ML Of 0350M Ammonia Solution. The p-Block Elements.
By signing up youll get. 2NH3aq H2SO4 aq -- NH42SO4aq a. Sulfuric acid is used in the manufacture of paints detergents and fertilisers.
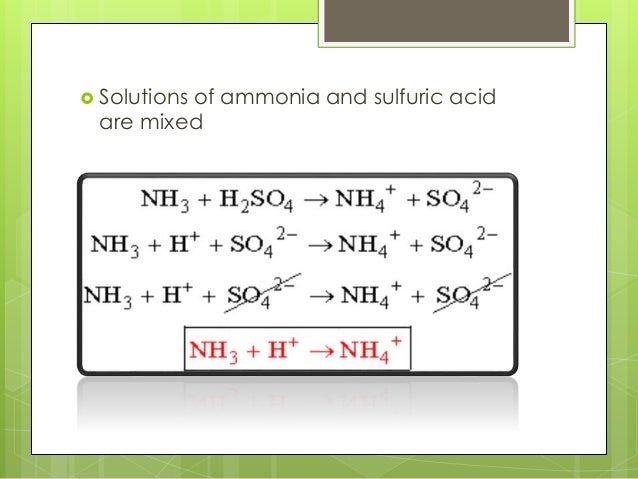
Write the balanced reaction of ammonia with sulphuric acid. 1 Sulfuric acid is a strong base that immediately dissociates when put in the water while ammonia is a weak acid that doesnt dissociate completely. 2 NH3 H2SO4 -.
The balanced equation for reaction between ammonia and sulfuric acid is. 2 NH 3 H 2 SO 4 NH 4 2 SO 4 A mixture of ammonia gas and water vapor is introduced into a reactor that contains a saturated solution of ammonium sulfate and about 2 to 4 of free sulfuric acid at 60 C. Balanced equation for the reaction between ammonia and sulphuric acid is2N H 3 H 2 S O4 N H 4 2 S O4.