Looking Good What Are Skeletal Equation

MnO4 Cr2 Water appears in the balanced equation as a for neither reactant product neither with a coefficient of Enter 0 Which species is the oxidizing agent.
What are skeletal equation. When the following skeletal equation is balanced under acidic conditions what are the coefficients of the species shown. A skeleton equation is when your chemical reaction is written with the chemical formulas that represent each substance that takes part in the reaction. Dark and Deadly collection Book 1 Coming Soon.
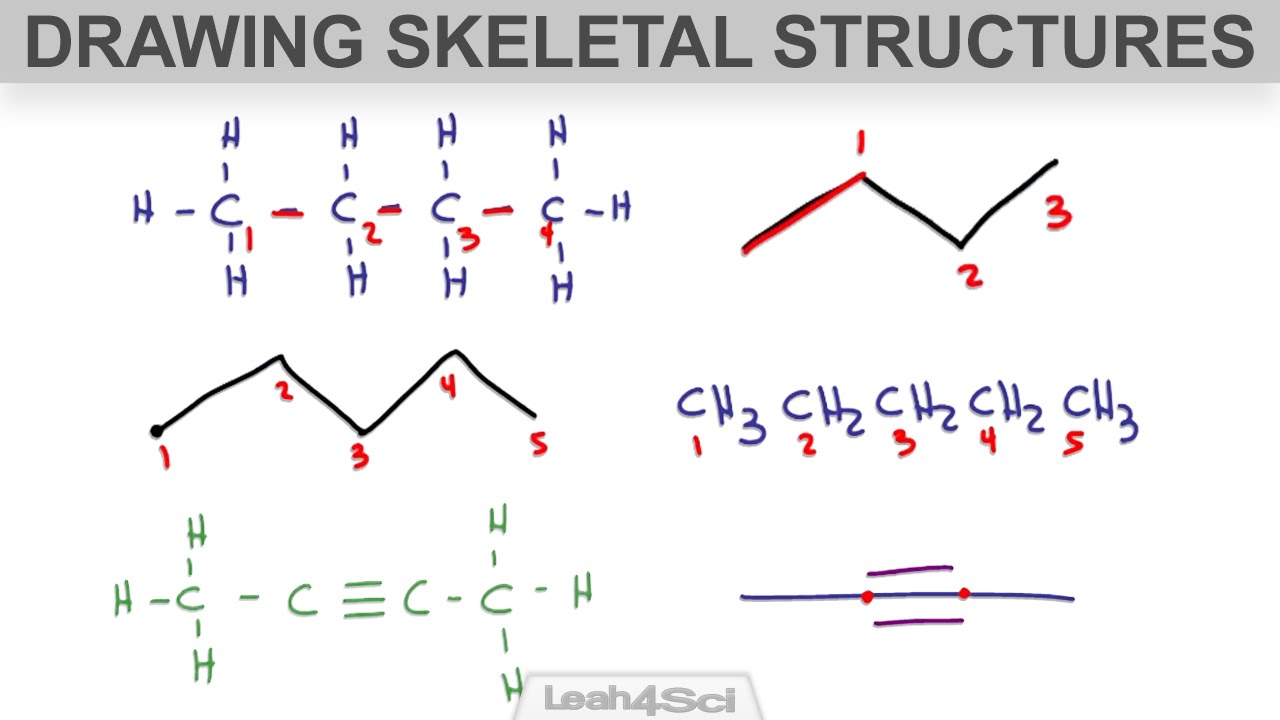
These equations are not needed to be balanced. An unbalanced equation is a chemical equation in which the total number of atoms of each element on the reactant side is not equal to the number of atoms of the same element on the product side. Drawing the Structural Formula Given the Skeletal Formula.
Recall that the main idea behind the skeletal decomposition is that under certain conditions we can identify prolific genealogies in the population and by immigrating non-prolific mass along the trajectories of these prolific genealogies we can recover the law of. When the following skeletal equation is balanced under acidic conditions what are the coefficients of the species shown. As carbon forms the backbone of organic molecules the first step towards constructing a skeletal formula can be to redraw the structural formula without writing in the letter C.
1 Pb 1 HNO3-1 Pb2 1 NO Water appears in the balanced equation as a reactant reactant product neither with a coefficient of O Enter for neither Which species is. This equation is also called a skeletal equation. Skeletal chemical equation is a representation of a chemical reaction using chemical formulae of reactants.
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. What is meant by skeletal chemical equation. Fe Au Co Br C O N F.
In skeletal formulae many of the carbon atoms and many of the hydrogens attached to carbon atoms are not drawn in explicitly. Mg O. A chemical equation in which the number of atoms of reactants are equal to the number of atoms of products is called a balanced equation.