Breathtaking What Type Of Change Is Rusting Of Iron

Corrosion of Iron F eI ron O2 F e2O3rust.
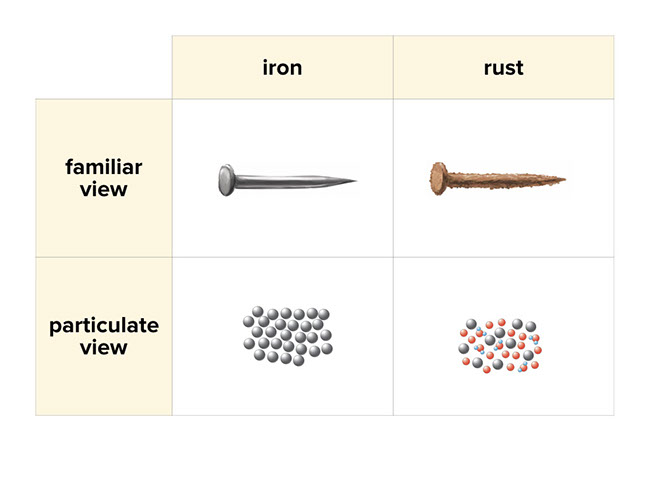
What type of change is rusting of iron. Rusting is an example of a chemical change. Chemical Reaction of Rusting is as follows. Rusting of iron refers to the formation of rust a mixture of iron oxides on the surface of iron objects or structures.
Rust consists of hydrated iron III oxides Fe2O3. A chemical property describes the ability of a substance to undergo a specific chemical change. What type of change is iron rusting.
It is usually a red oxide. Rusting is an example of a chemical change. Iron water oxygen.
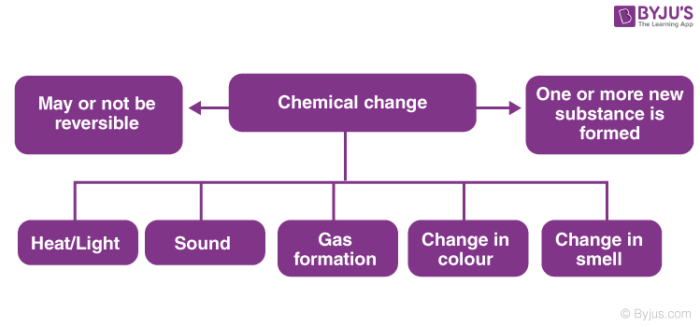
A chemical property of iron is that it is capable of combining with oxygen to form iron oxide the chemical name. It is an irreversible change. Rusting is an oxidation reaction.
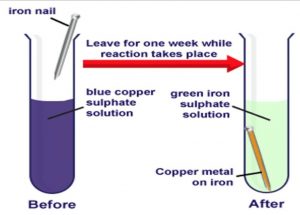
Is iron rusting in the presence of moist air a physical change. Further because the reaction is irreversible. The underlying equation underlying rusting is Fe O2 H2O - Fe2O3 and because a new substance is created this is indeed a chemical change.
Given sufficient time any iron mass in the presence of water and. The oxygen and water in air react with the metal to form the hydrated oxide. Rust is essentially iron oxide or sometimes iron alloys.