Sensational Sodium Hypochlorite And Hydrochloric Acid Balanced Equation

This equates to 04 to.
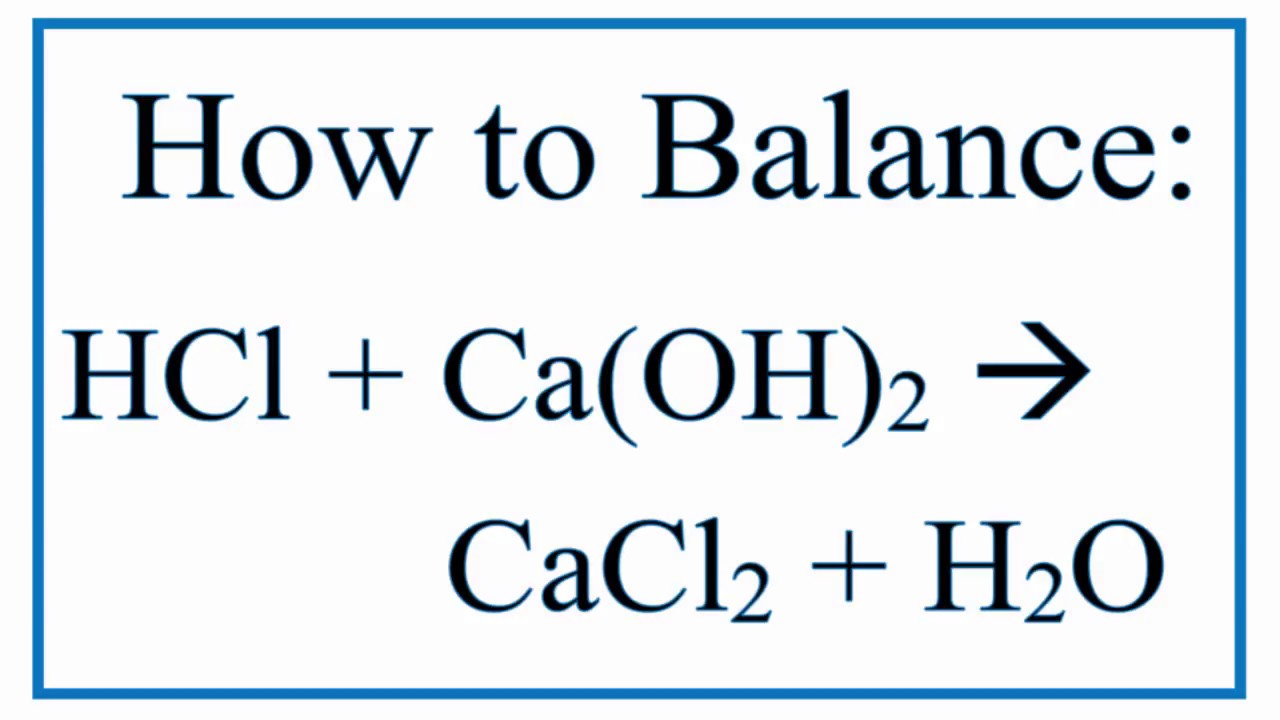
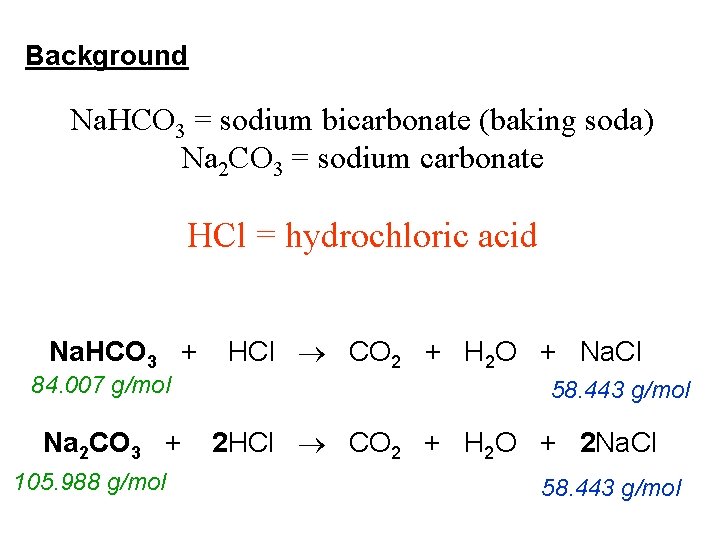
Sodium hypochlorite and hydrochloric acid balanced equation. Asked Jan 19 2018 in Science by Rohit Singh 643k points acids bases and salts This is a neutralization reaction between an acid HCl and a base NaHCO3 The products of most. Sodium hydroxide is a strong base. HClNaOH-NaClH_2O The reactants are hydrochloric acid HCl and sodium hydroxide NaOH and the products are sodium.
HOCl -- H OCl-The main difference between chlorine gas and hypochlorite compounds is that gas tends to decrease pH which favors hypochlorous acid formation. If there was any unreacted sodium hydroxide left in your sodium hypochlorite it would just make more bleach. A MMS1 refers to Jims original formula of 224 Sodium Chlorite solution activated with an equal amount of 50 Citric Acid solution.
Hypochlorite compounds tend to increase pH with the occurrence of sodium hydroxide which favors. NaOClaq HClaq HOClaq NaClaq. The balanced equation that shows the stoichiometric reaction between sodium sulfide and hydrochloric acid to produce hydrogen sulfide gas and sodium chloride is Na2S 2HCl 2 NaCl H2S.
2 Na s Cl 2 g 2 NaCl s NaCl is salt of strong acid and strong base. Sodium hypochlorite is made by bubbling chlorine through a solution of sodium hydroxide. A ionic equation of a reaction isnt much different from a regular reaction equation except that you write the ions separately.
Chemical Balanced Equation In hypochlorite ion chlorine atom is in the 1 oxidation state. Sodium Carbonate and Hydrochloric Acid Reaction Na 2 CO 3 HCl. In other words sodium hypochlorite and hydrochloric acid make saltwater and chlorine gas.
Sodium hydroxide is a base because it has hydroxide ionThe chemical formula for sodium hydroxide is NaOHIt is a strong base. Mechanism of sodium hypochlorite and hydrochloric acid 3 The overall reaction for the reaction between NaClO and HCl is. A pH between 12 and 13 gives the most stable solution.