Best Oxidation State Of Hcl
Hydrochloric Acid HCl Recycle to Chlorine by the Sumitomo Catalytic Oxidation Process Marianna Asaro Executive Director of Industrial Chemistry and Catalysis Abstract Hydrogen chloride HCl is produced as a byproduct in various chemical chlorination processes and as burner acid in.
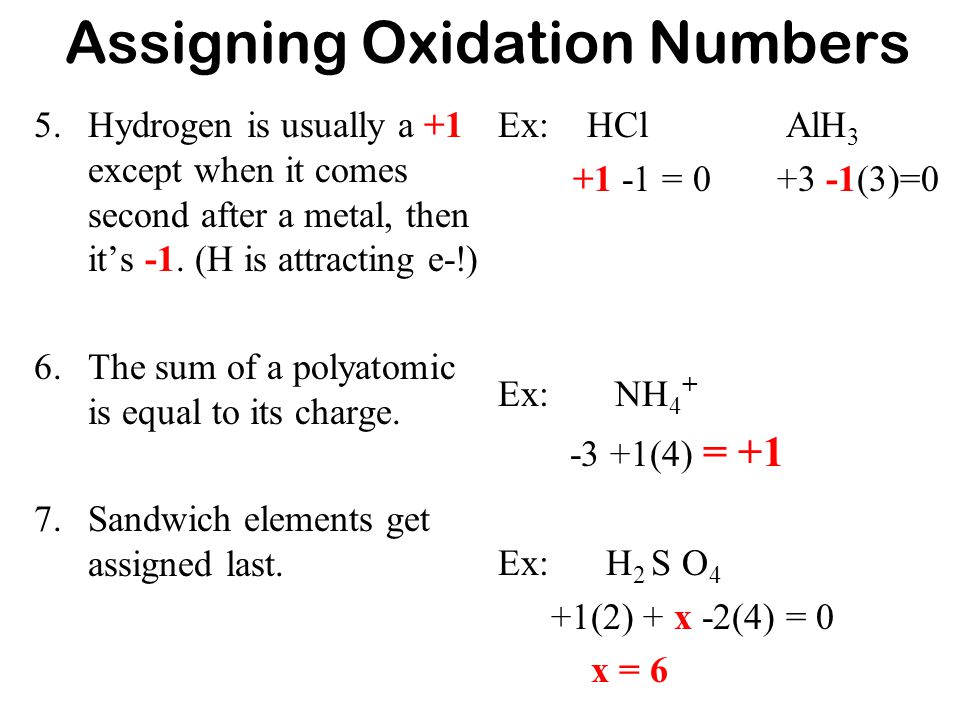
Oxidation state of hcl. The total of oxidation states of neutral molecule is. The oxidation state of Cl is -1. Other halogens usually have oxidation states of 1 as well except when combined with oxygen or other halogens.
The oxidation state of fluorine in chemical compounds is always 1. So the oxidation number of the compound HCl is zero. Assume the oxidation state of Al as x.
What Is The Oxidation State Of The Underlined Atom. Alternatively the sum of the oxidation states in a neutral compound is zero. 388 Chapter XIII THE OXIDATION OF HYDROGEN CHLORIDE The oxidation of hydrogen chloride the Deacon process.
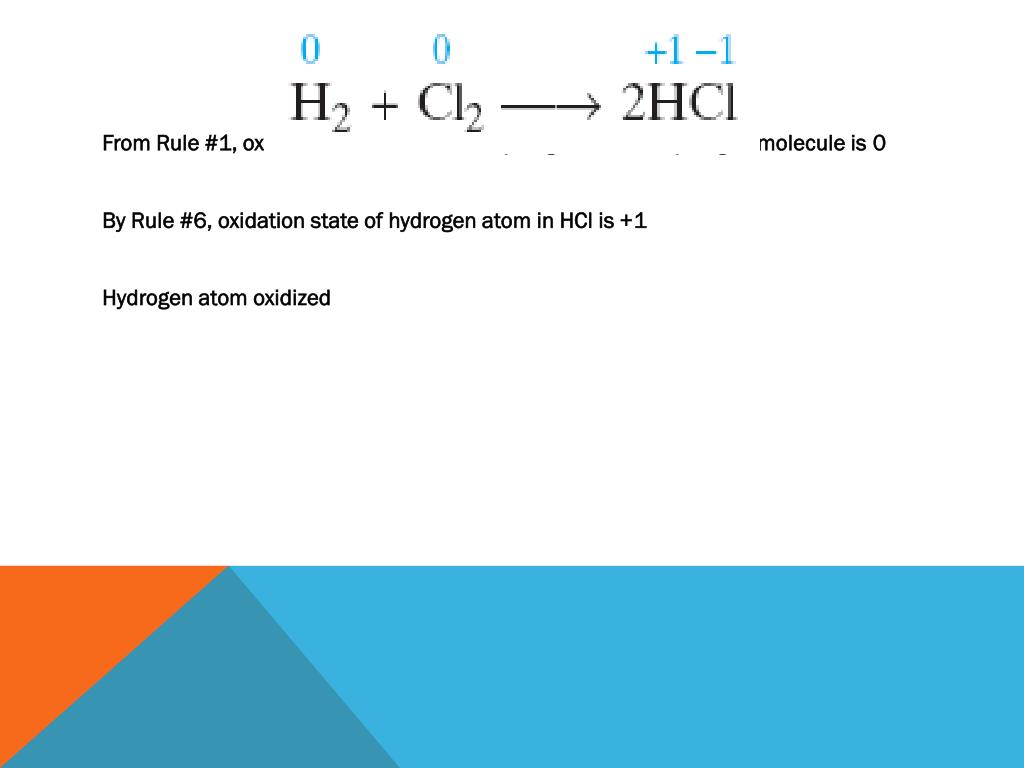
Hydrochloric Acid HCl As per the rules discussed above the oxidation state of a group 17 element halogen in a diatomic molecule is -1. Because Group 1 metals always have an oxidation state of 1 in their compounds it follows that the hydrogen must have an oxidation state of -1 1 -1 0. Chemistry questions and answers.
In a compound hydrogen has an oxidation number of 1. Henry in 1826 1. You have the oxidation number of Al is 0 H in HCl is 1 AlCl3 is 3 H in H2 is 0.
122 rows The oxidation state sometimes referred to as oxidation number describes the degree of. KMnO4 is a strong oxidizing agent which will oxidize vanadium to its maximum oxidation state. The oxidation state of O is -2 and 1 for H so as HClO is neutral overall the oxidation state of Cl in HClO is 1.