Out Of This World Damp Iron Wool Experiment
In the experiment students will investigate the effects of different environments on the corrosion of steel wool iron.
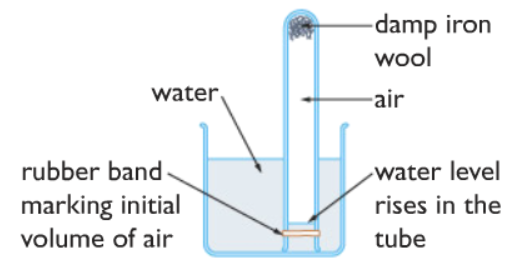
Damp iron wool experiment. Water damp iron wool Which statement explains this rise. F e H X O A c X F e X 2. Fluff the wool up a little bit to make it easier for oxygen to access the iron.
4Fe s 3O 2 g à 2Fe 2 O 3 s 5. The oxygen bonds to the metal and a new compound is. In how quickly iron will rust.
The set-up was left un-disturbed for three. The rusting of iron is actually a good example of the process of corrosion. Even if you dont want to stud.
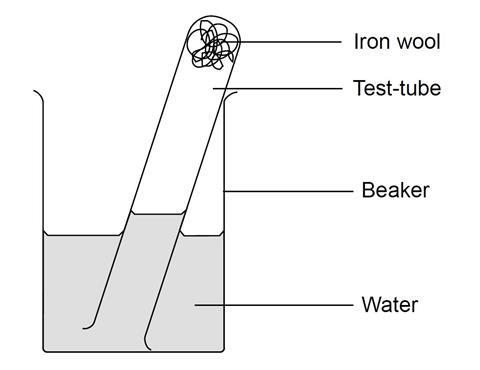
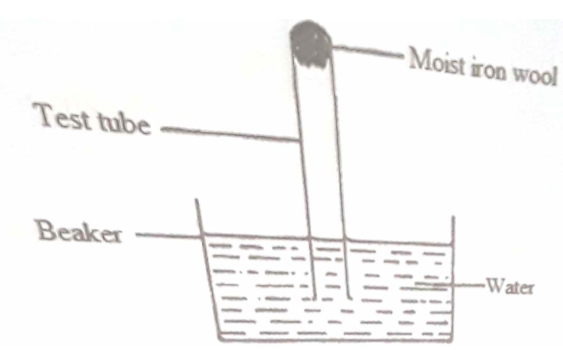
In an experiment a gas jar containing some damp iron fillings was inverted in a water trough containing some water as shown in the diagram below. Moist iron wool Water. In the first experiment the heat from the glowing.
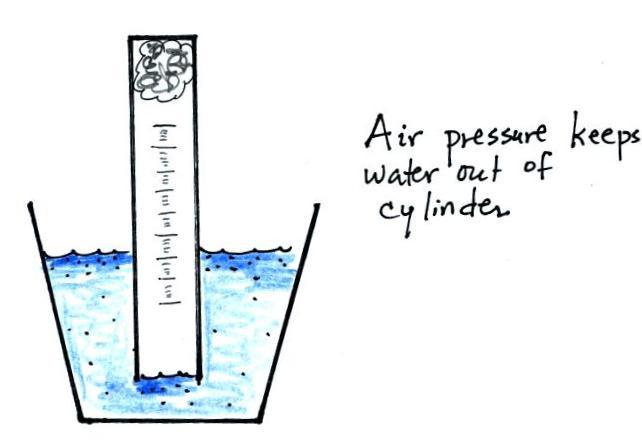
Under wet conditions iron will rust more quickly. State and explain the observation made after three days. As a result water will rise up in the jar to take the place of.
The iron is gradually eaten away as it reacts slowly with the oxygen. Ideas for Chemistry Projects Involving Iron Rusting. A Iron oxide has been formed.