Unbelievable Examples Of Endothermic Reaction Class 10

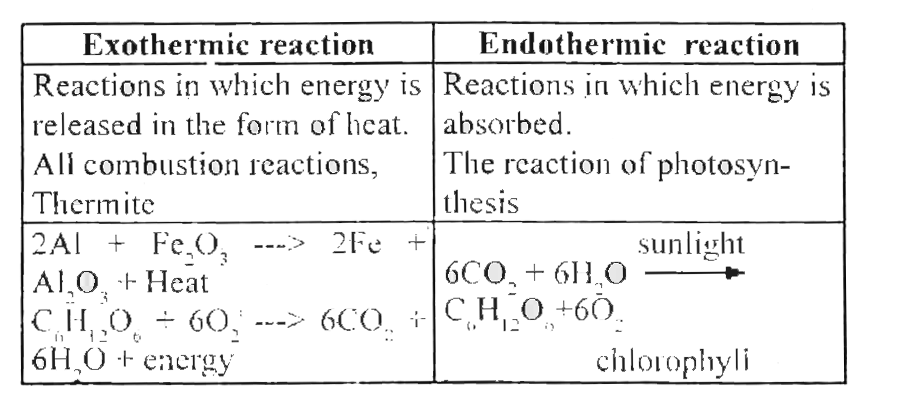
In an exothermic reaction change in enthalpy ΔH will be negative.
Examples of endothermic reaction class 10. The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. Endothermic Reaction For example. Chemical Reactions Equations given the following worksheet with figure18 given on page 10 of class of lead nitrate is an example of x reaction This is a useful class practical to introduce energy changes in chemical reactions.
The decomposition of carbonic acid in soft drinks which can be represented by the chemical equation H 2 CO 3 H 2 O CO 2. A chemical reaction that works only if heat is retained is a case of a reaction that would be portrayed as endothermic. Converting frost to water vapor melting boiling and evaporation in general are endothermic processes.
Compared to an endothermic reaction where energy is absorbed the. When ammonium chloride NH 4 Cl is dissolved in water an endothermic reaction takes place. Some common examples of decomposition reactions are provided below.
The definition of an endothermic reaction is that it is a chemical reaction that is usually accompanied by the retention of warmth or heat or a living being that produces warmth or heat to keep up its temperature. All the decomposition reactions require energy to take place. An exothermic reaction is a reaction in which energy is released in the form of light or heat.
The chemical equation can be written as follows. The reactions which occur by the absorption of heat energy either in the form of light or electricity are called endothermic reactions. Thus in an exothermic reaction energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction.
ThoughtCo Hilary Allison. The electrolysis of water to yield hydrogen and oxygen. Ammonium nitrate is dissolved in water.









.PNG)
