Unbelievable Skeleton Equation Chemistry Definition

This equation is also called a skeletal equation.
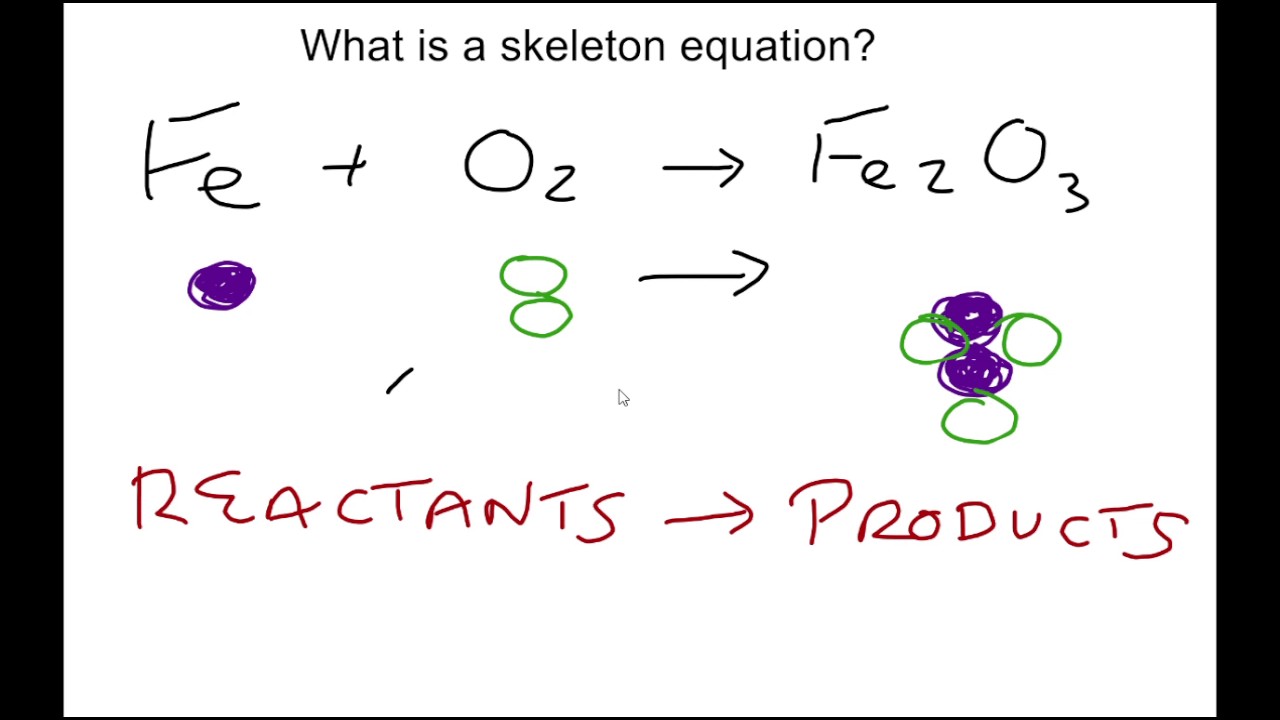
Skeleton equation chemistry definition. Later it has to be balanced by appropriate number of molecules. A chemical equation that does not indicate the relative amounts of the reactants and products. An unbalanced equation is a chemical equation in which the total number of atoms of each element on the reactant side is not equal to the number of atoms of the same element on the product side.
An expression representing a chemical reaction. The formulas of the reactants on the left are connected by an arrow with the formulas for the products on the right Book Definition of. In essence it is identical to a word equation except that the names of the reactants and the products are substituted by their chemical symbols.
The skeleton structure of a covalent molecule can often be determined by considering the valences of the constituent atoms. Writing proper formulas for the species in chemical reactions equations. Usually the atom which forms the largest number of bonds is found in the center of the skeleton where it can connect to the maximum number of other atoms.
The next step is Balancing Chemical EquationsTable of C. Hypochlorous acid has the molecular formula HOCl. Use uppercase for the first character in the element and lowercase for the second character.
Skeletal chemical equation is a representation of a chemical reaction using chemical formulae of reactants and products. Give the skeleton chemical equation and balanced equation of this. A skeleton equation is a way of using formulas to indicate the chemicals that are a part of the chemical reaction.
The key difference between balanced equation and skeleton equation is that balanced equation gives the actual number of molecules of each reactant and product involved in the chemical reaction whereas skeleton equation gives only the reactants of the reaction. Video was made using Screencast-O-Matic. A chemical equation is a representation of a chemical reaction.