Breathtaking Ethane Plus Oxygen Balanced Equation

For example using ethane C2H6.
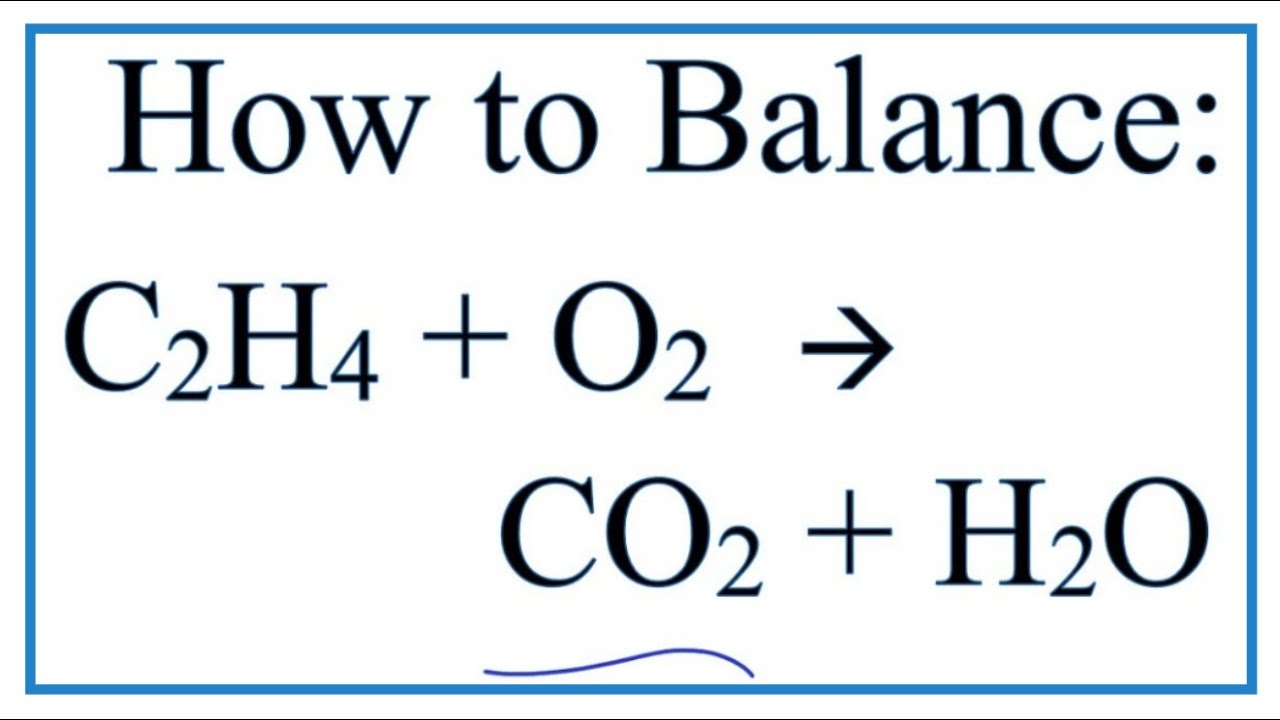
Ethane plus oxygen balanced equation. C2H2 O2 2 CO2 H2O need 25 O2s to balance the Os Ethane burn in the presence of oxygen to form carbon dioxide and water and release of energy in the form of heat and light. To balance the chemical equation we add coefficients before each chemical. When we find the right.
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. How to balance the chemical equationC2H6 O2 CO2 H2OEthaneoxygencarbon dioxidewater. The question gives us the products and reactants.
B Write the balanced equation for the reaction. The balanced equation will appear above. C2H6 35O2 ----- 2CO2 3H2O.
D What is the total mass of products expected from the combustion of 136 g of ethane. Fuel O2 CO2 H2O You would then balance the chemical equation. In a balanced reaction.
The anwser is 2 C2H6 7 O2 4 CO 2 6 H2O. C What mass of O 2 in grams is required for complete combustion of 136 of ethane. Ethane gas C2H6 C 2 H 6 reacts with oxygen gas to produce carbon dioxide gas and water vapor according to the reaction shown below.
To construct a symbol equation for the incomplete combustion of a fuel you need to read the question to see whether carbon monoxide CO or carbon C is produced. Ethane has a chemical formula of C2H6. The number before each of.