Brilliant Hydrogen Chloride Plus Ammonia

Consequently the molecule has a large dipole moment with a negative partial charge δ at the chlorine atom and a positive partial charge δ at the hydrogen.
Hydrogen chloride plus ammonia. The theft leaves chloride alone and negative. A combination of ammonia and hydrogen peroxide is often used in the process of bleaching hair. Hydrogen chloride is added to a buffer solution of ammonia NH3 and ammonium chloride NH4Cl.
Ammonium chloride is prepared commercially by combining ammonia NH 3 with either hydrogen chloride gas or hydrochloric acid water solution. What exactly is accepting the lone pair of electrons on the nitrogen. Then hydrogen chloride reacts with basic ammonia gas to produce ammonium chloride which is a solid white smog.
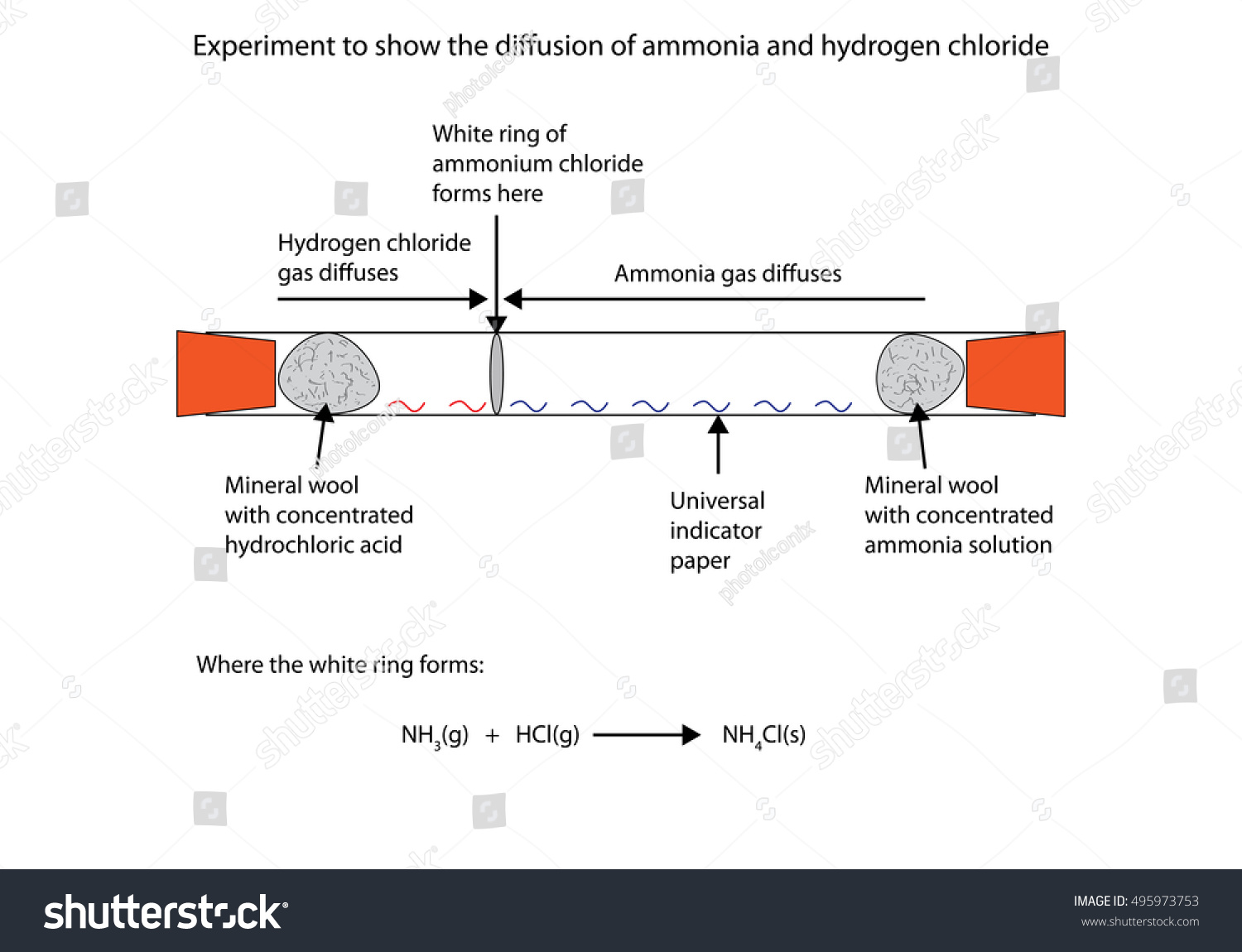
In water the reaction between ammonia NH 3 and hydrogen chloride HCl is a textbook example of acid-base chemistry. HCl and NH 3 molecules diffuse through the air towards each other. Lewis Acids and Bases.
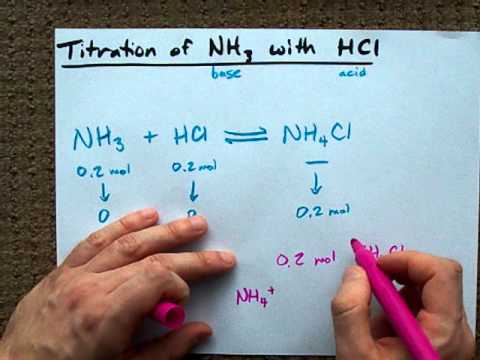
Produced hydrogen chloride vapor can behave as an acidic compound can release H ions in the water. NH3 g HCl g --- NH4Cl s Two 200-L flask at 25 degrees Celcius are connected by a valve. Lewis proposed a generalized definition of acid-base behavior in which acids and bases are identified by their ability to accept or to donate a pair of electrons and form a coordinate covalent bond.
Hydrogen Chloride in gaseous form is a covalent compound and a Lewis acid which for example can react with Ammonia gas to produce Ammonium Chloride. Ammonia and hydrogen chloride react to form solid ammonium chloride. The cotton wool with hydrochloric acid gives off hydrogen chloride molecules HCl.
To happen this second step reaction ammonia is required. A An aqueous solution of chlorine is acidic as it dissolves in water to form hydrochloric and hypochlorous acids. B Hydrogen chloride and ammonia c Hydrogen and oxygen d AgClSilver chloride e Aqua regia f Fountain experiment g Hydrogen chloride gas.