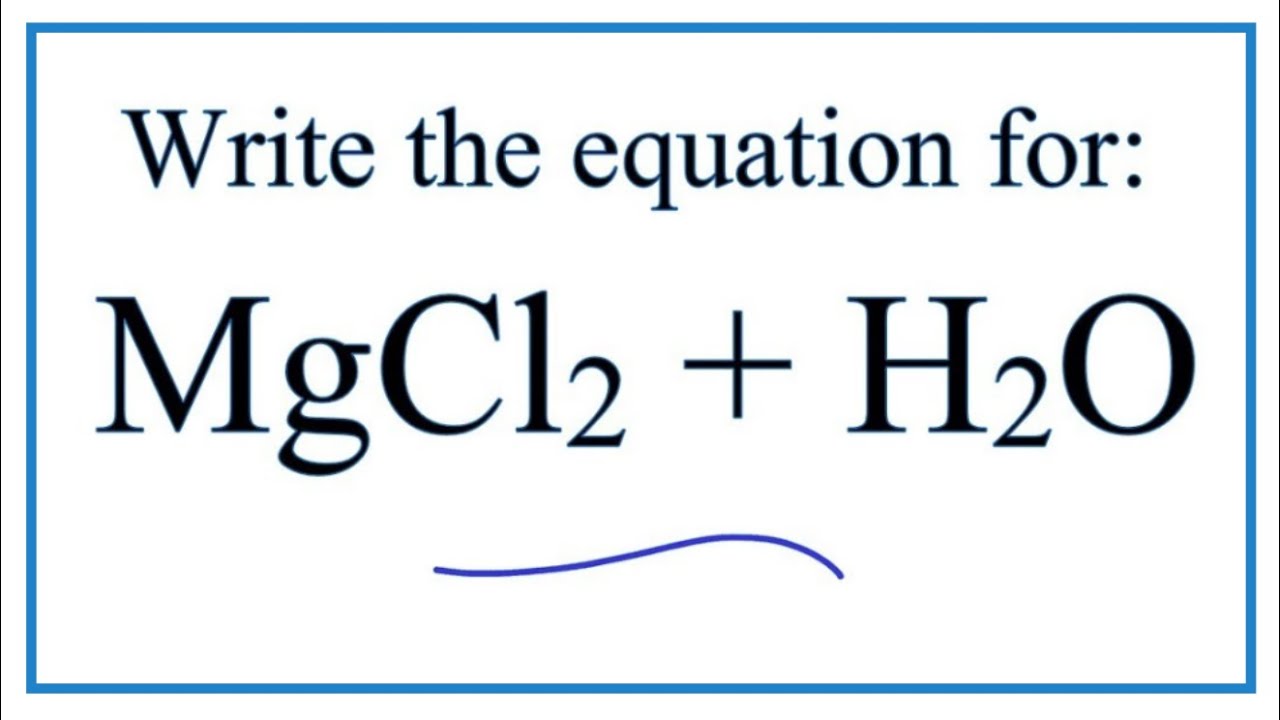Neat Magnesium Chloride Reaction With Water

However Sorel cement is attacked by water and it.
Magnesium chloride reaction with water. When exposed to steam magnesium changes from magnesium to magnesium oxide and hydrogen. Hydrolysis of a phenylmagnesium halide produces benzene with a potential release of hydrogen halide vapors such as hydrochloric acid HCl vapors 3. As an approximation the simple ionic chlorides sodium and magnesium chloride just dissolve in water.
This reaction takes place at a temperature near 500C. Mg OH2 H2CO3 H2O Mg HCO32 We do this because magnesium bicarbonate is by a long stretch the most absorbable form of magnesium available. Mix 33 grams of Greenway Biotech Magnesium Chloride USP with 1 liter of filtered water or juice.
Magnesium chloride dissolves in water to give a slightly acidic solution with a pH of approximately 6. Does magnesium chloride react with water at room temperature. For example magnesium chloride and magnesium oxide Sorel cement react very rapidly in water to form MgOMgCl2 11H 2 O and Mg OCl 2.
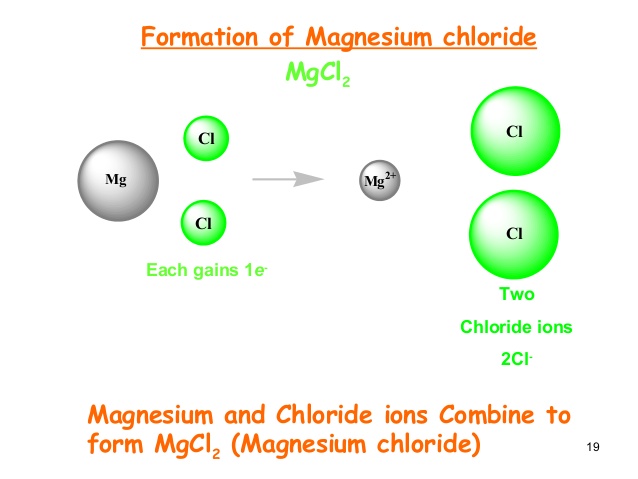
Magnesium dichloride and its salts are ionic halides and appear as thin white to gray granules. CID 5462224 Magnesium Date s. The other chlorides all react with water in a variety of ways described below for each individual chloride.
It has a role as a Grignard reagent. Every shot about 45 milliliters or 15 ounces of this solution makes one dosage of Magnesium supplement. Ethylmagnesium chloride is an alkylmagnesium halide.
You are going to react the magnesium hydroxide of MOM with the carbonic acid of the carbonated seltzer to yield water H2O and magnesium bicarbonate Mg HCO32. MgCl 2 can be extracted from sea water or brine and is chemically named as Magnesium Chloride. When magnesium ions are solvated from the solid lattice there is enough attraction between the 2 ions and the water molecules to form coordinate dative covalent bonds between the magnesium ions and lone pairs on surrounding water molecules.