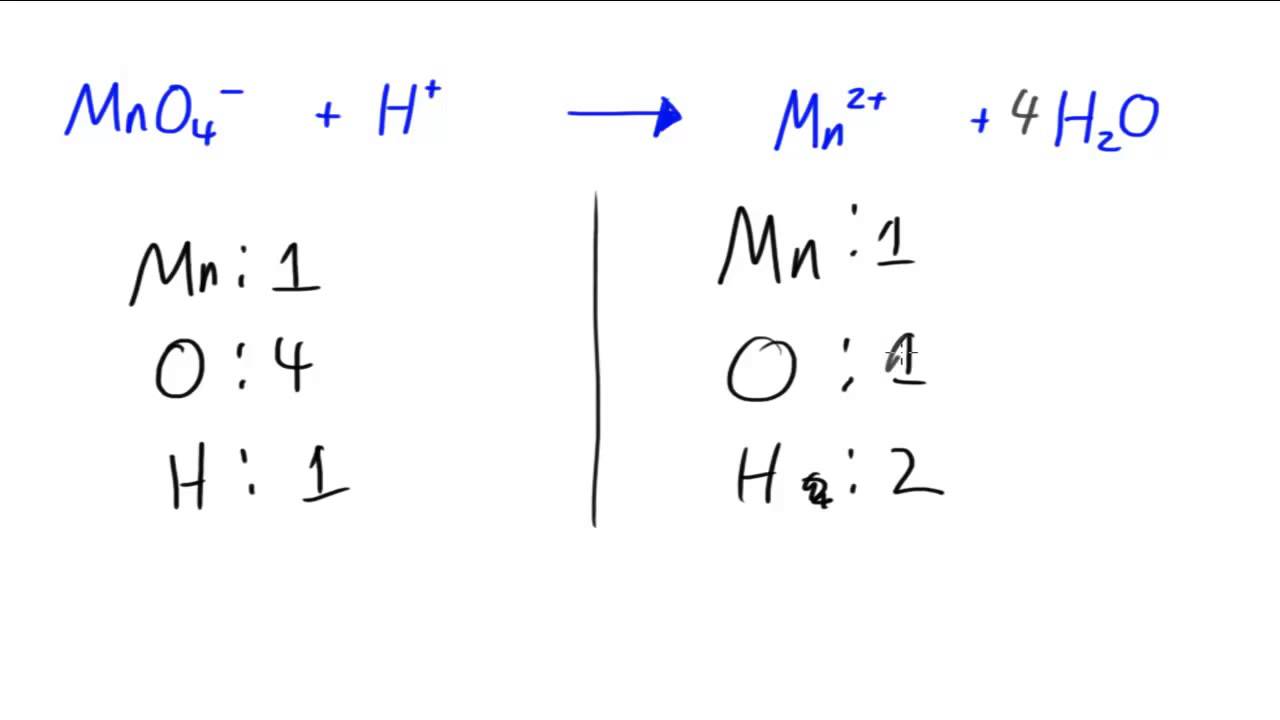
Glory Chemical Equation Balancer With Charges

Instructions on balancing chemical equations.
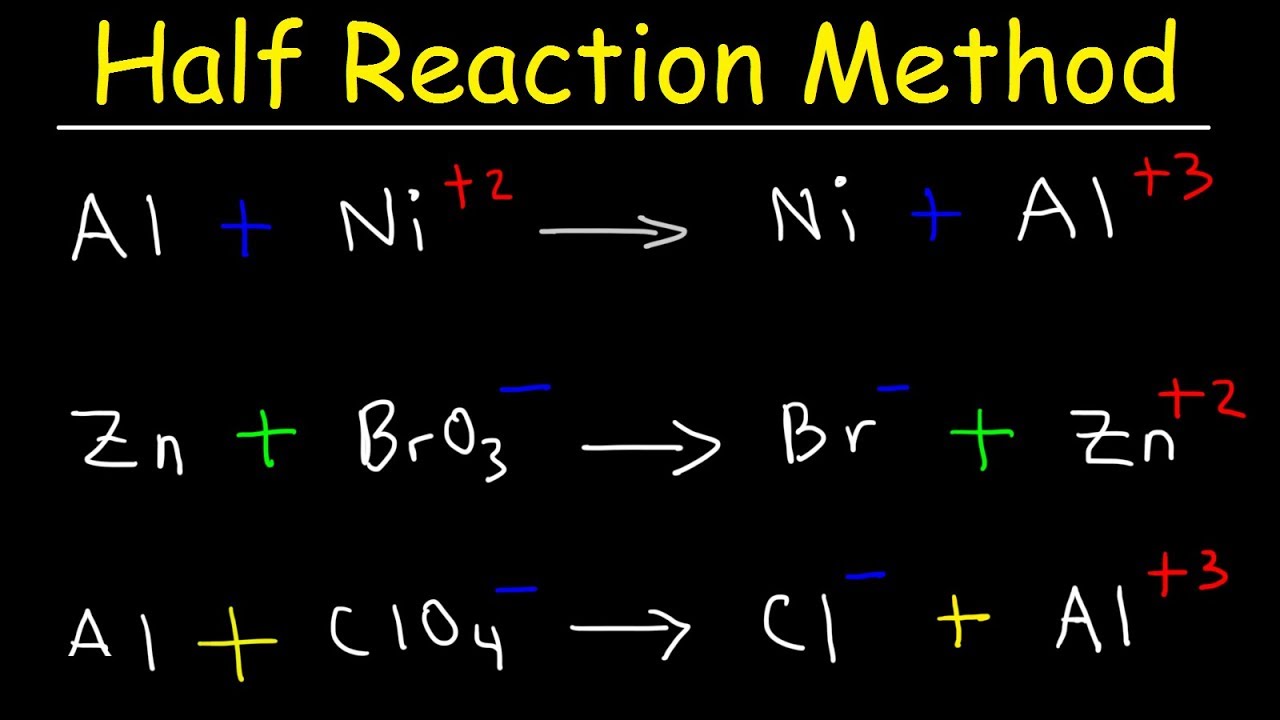
Chemical equation balancer with charges. Just as general equation there are two atoms of sodium 1Na212 2 and two atoms of chlorine 1Cl2122 which reacts with each other to form 2 molecules of NaCl 2NaCl2Na212 2C1212 Hence proved that the process of balancing chemical equations involves balancing the equal number of atoms of reactants and products. A chemical equation is the representation of the chemical reactions. Instructions To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
Because the sum of the atoms on the left side of the equation is equal to the sum of the same atoms on the right side of the equation and the charges on both sides are equal the chemical reaction calculator write a balance equation 3 O 2 4 F e 2 F e 2 O 3. Chemical equation balance app is the best equation balancer app and also work as chem calculator or chemical balancer. Na H 3 O acetate OH - Charge balances get interesting when one of the ions has a charge greater than one.
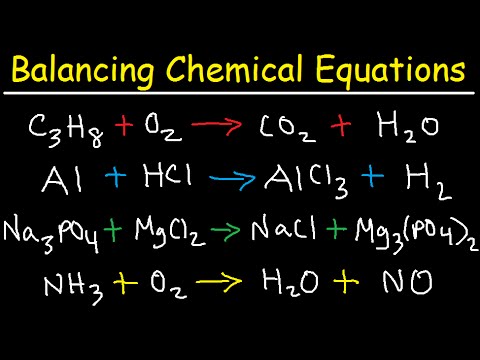
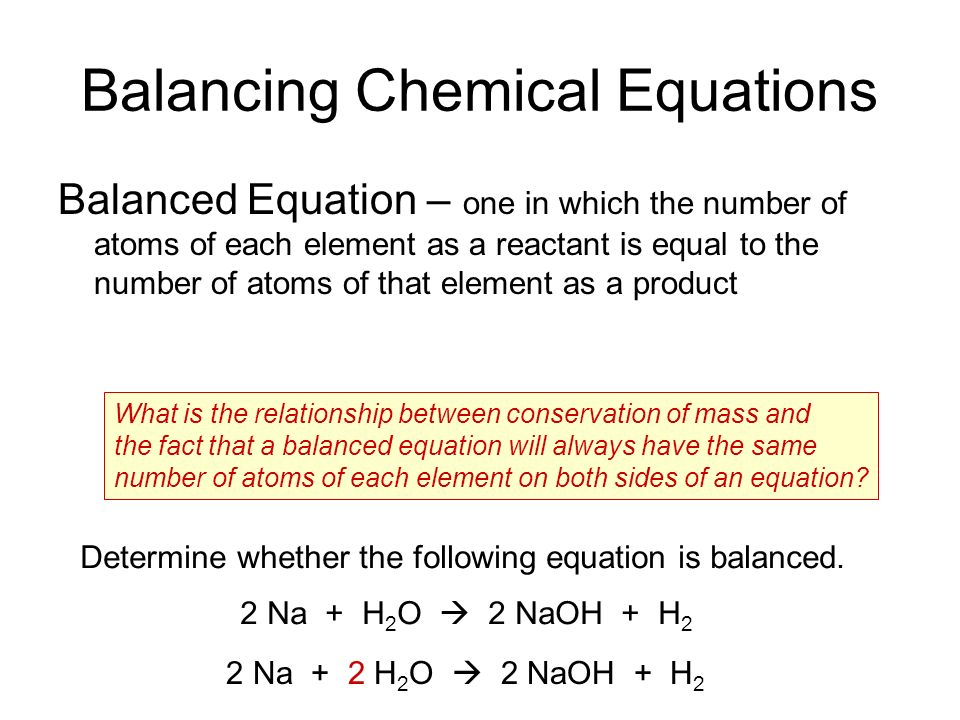
Balancing chemical equation is the process of equalising the number of each element in the reactants to the products. The LHS consists of the reactants and the RHS consists of the products. What is the charge balance equation for a solution of Acetic Acid.
HAC H Ac is the reversible chemical equation for acetic acid which is a weak monoprotic acid. Co - cobalt and CO - carbon monoxide. The balanced equation will appear above.
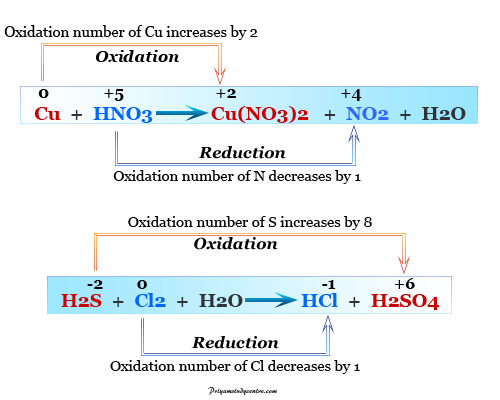
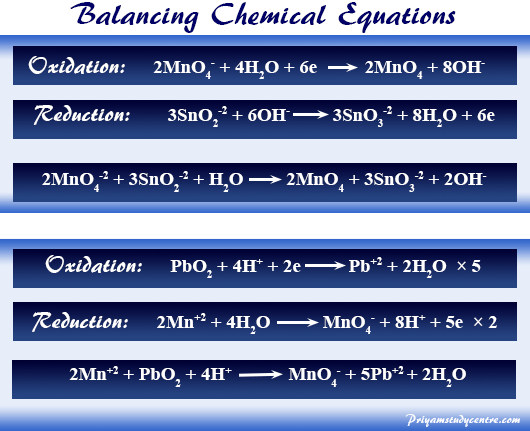
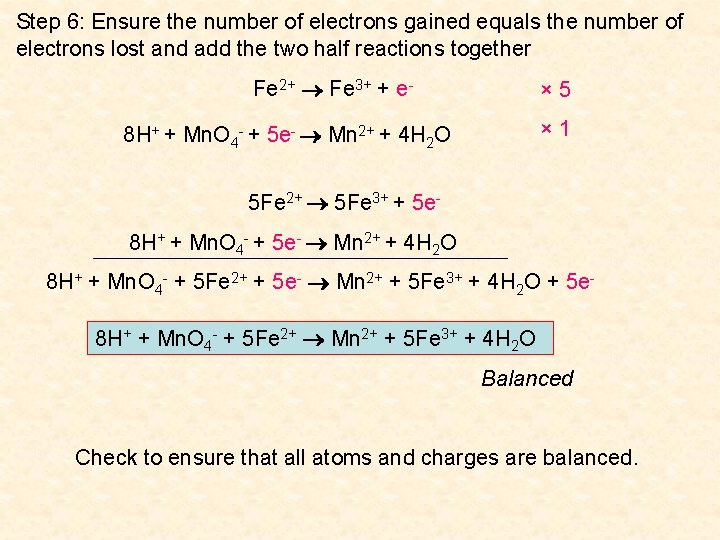
In other words the mass and charge on both sides of the reaction are equal. It is an online tool which works digitally and provide quick results. A balanced equation is a chemical reaction equation in which the total charge and the number of atoms for each element in the reaction are the same for both the reactants and the components.
O H OH- OCI- O H OH- AC- O H OH- AC- O H OH- Ac- Question 4 6 pts Using 3 equations and 2 assumptions we can solve for the equilibrium Select. The charge balance must account for all positively charged sodium and hydronium ions and negatively charged acetate and hydroxide ions species in solution. Introduction of Chemical Equation Balancer.