Nice Chemical Equation For The Formation Of Rust

Steel is an alloy made of iron and carbon.
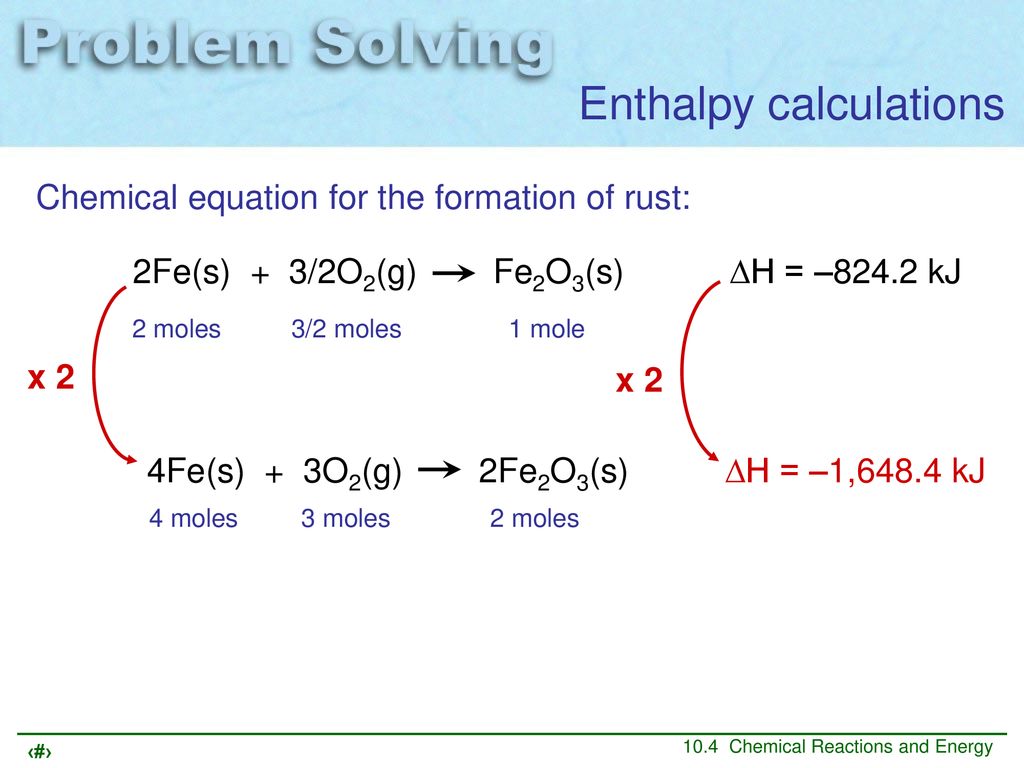
Chemical equation for the formation of rust. 4Fe 3O2 2Fe2O3. The chemical formula for rust is Fe2O3. Rust is an iron oxide a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture.
2Fes 2H 2 Ol O 2 g 2Fe 2 aq 4OH-aq. How many atoms are in H2O. The overall chemical equation for the formation of rust is.
Oxygen gas combustion and synthesis copper oxide. Fe 2 aq 2OH-aq FeOH 2 s. 2 Mg O 2 2 MgO.
From the equation for rusting you can see that four atoms of iron combine with three molecules of oxygen to form two molecules of iron oxide. Fe OH3 dehydrates to produce Fe2O3nH2O s or rust. Rusting occurs as the corrosion of iron in the alloy steel.
Rust is apparently a. Iron Water Oxygen Rust ie. The rusting process requires both the elements of oxygen and water.
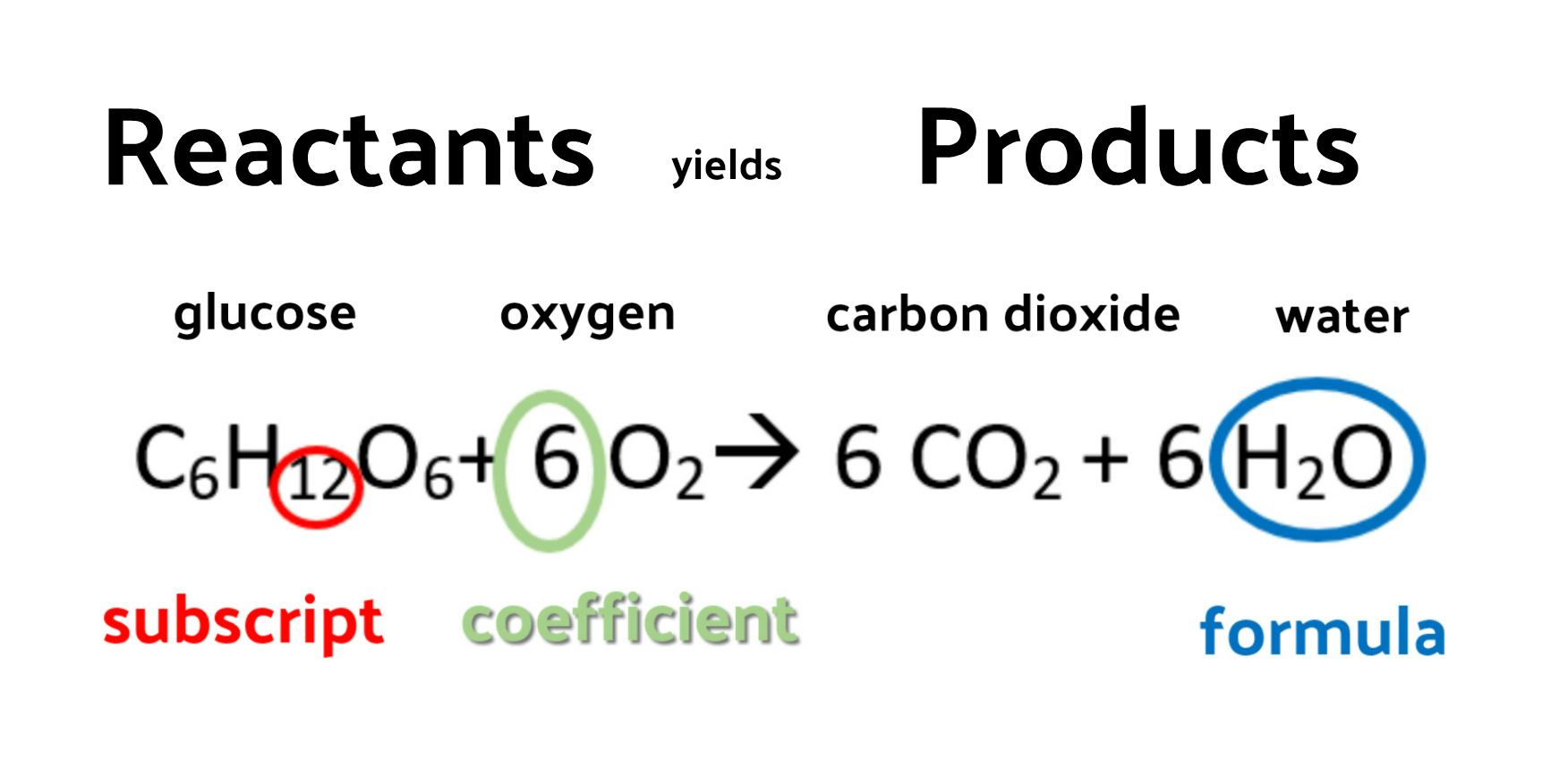
X H 2 O. Water is also required for this reaction to occur but because the total amount of water does not change it is not included in the equation. We can go further and translate the picture equation for the reaction between magnesium and oxygen to a chemical equation.