Great Chapter 8 Review Describing Chemical Reactions

List four metals that will not replace hydrogen in an acid.
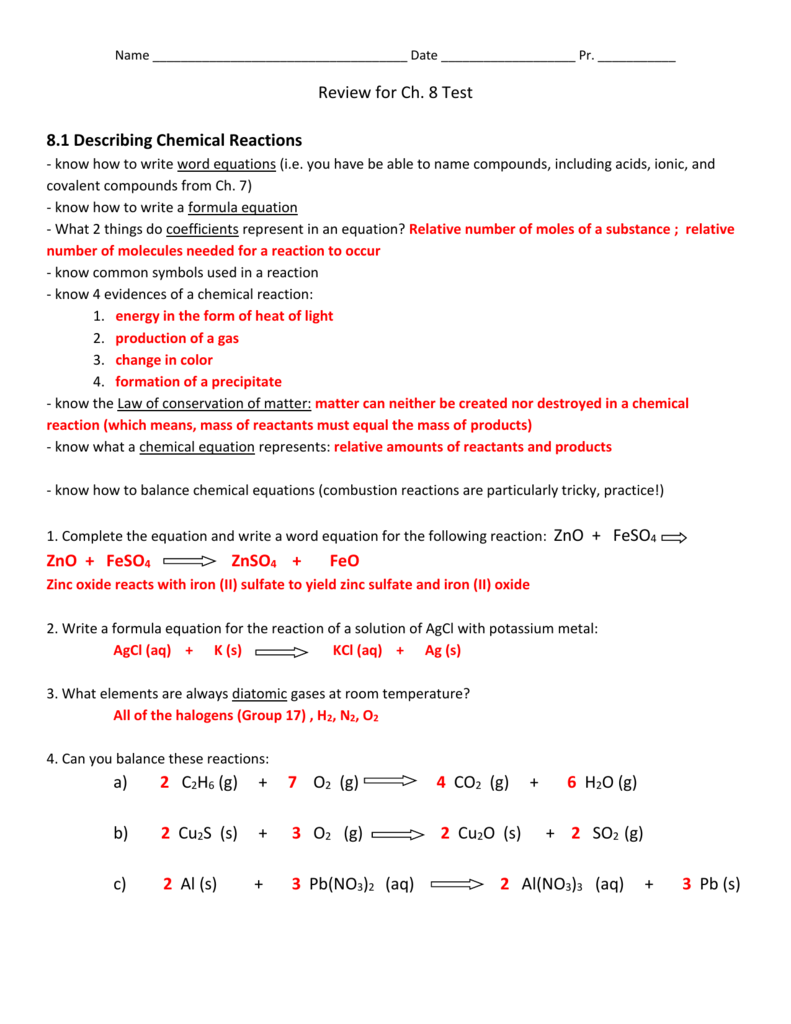
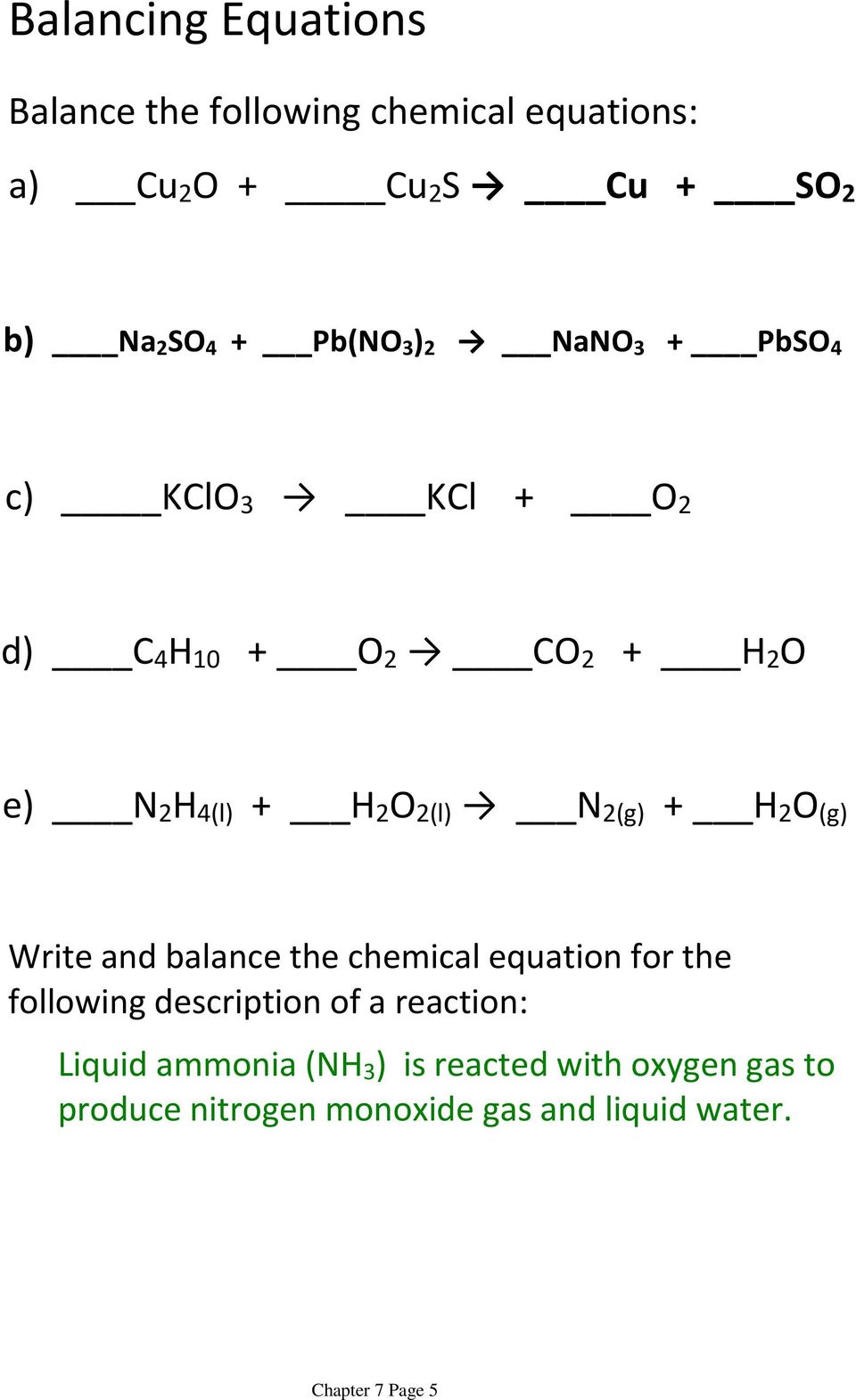
Chapter 8 review describing chemical reactions. Write a word equation and a formula equation for a given chemical reaction. List four metals that will not replace hydrogen in an acid. The mass of the products of the reaction C2H5OH 3O2 --- 2CO2 3H2O equals the mass of the reactants because the products.
Write a word equation and a formula equation for a given chemical reaction. CHAPTER 8 REVIEW Chemical Equations and Reactions SECTION 3 SHORT ANSWER Answer the following questions in the space provided. Choose from Cu Ag Au Pt Sb Bi and Hg.
Chapter 8 Energy and Chemical Reactions 105 Section 84 Chlorofluorocarbons. Access Free Chapter 8 Chemical Equations And Reactions Test Answer Key 8 Chemical Equations and Reactions Modern Chemistry 1 Chemical Equations and Reactions CHAPTER 8 REVIEW Chemical Equations and Reactions Teacher Notes and Answers Chapter 8 SECTION 1 SHORT ANSWER 1. Chapter 8 - Chemical Equations and Reactions 8-1 Describing Chemical Reactions I.
Knowledge about what products are produced in a chemical reaction is obtained by. Original substances entering into a chemical rxn B. Consider the metals iron.
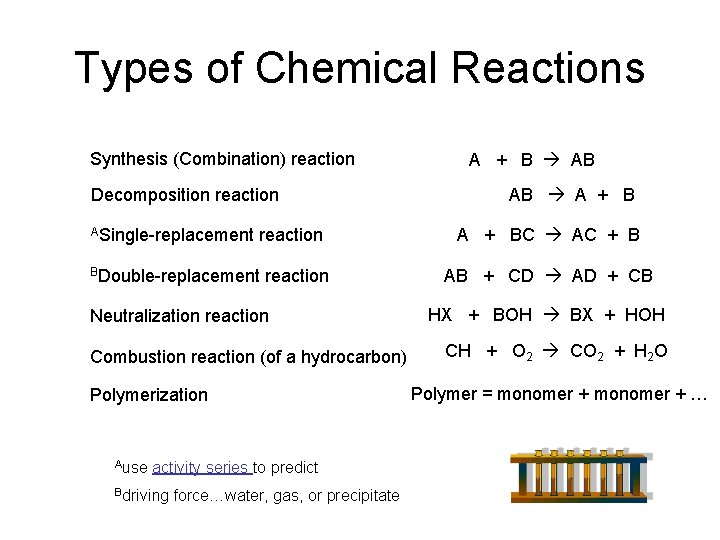
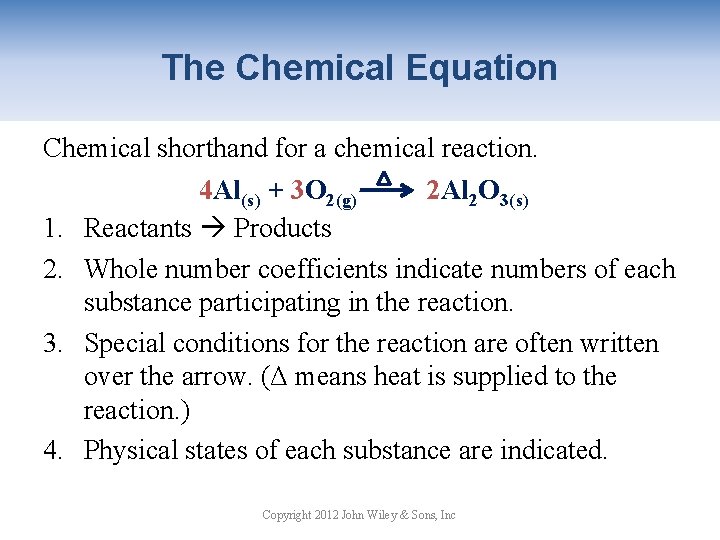
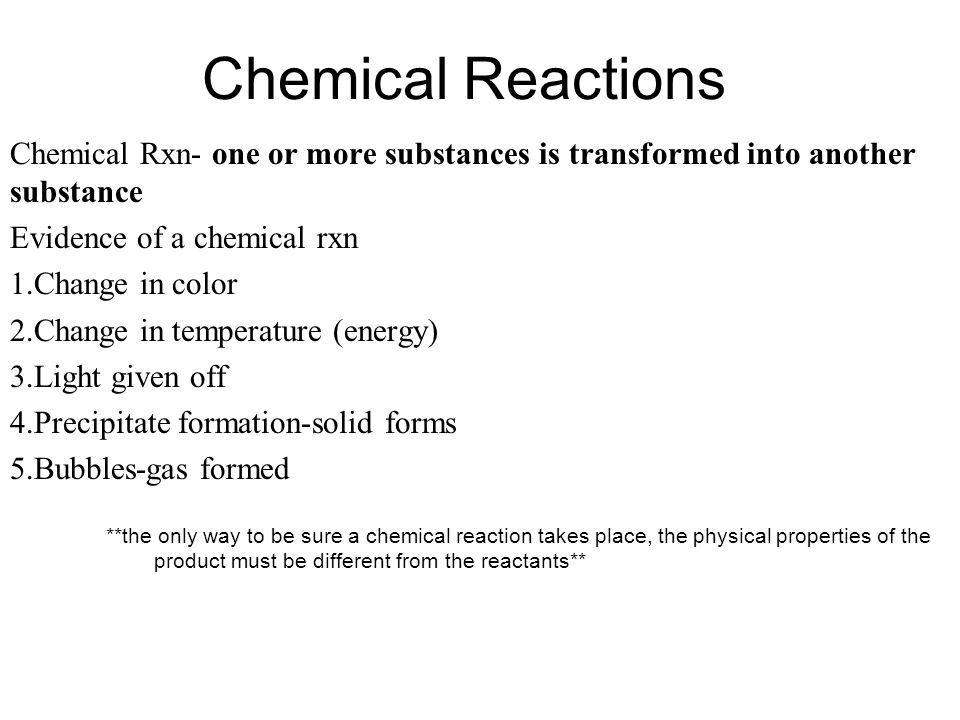
Choose from Cu Ag Au Pt Sb Bi and Hg. A representation of a chemical reaction using the chemical formulas of the reactants and products. List three observations that suggest that a chemical reaction has taken place.
Terms in this set 27 chemical equations. Section 81 Part I Objectives List three observations that suggest that a chemical reaction has taken place. Chapter 8 describing chemical reactions section 1 review answers Process that results in the interconversion of chemical species A thermite reaction using ironIII oxide.