Fabulous Balanced Chemical Equation Of Rusting Of Iron

Iron and pure neutral water dont react much.
Balanced chemical equation of rusting of iron. These reactions are called combustion reactions. Oxidation of Fe to Fe3 gives you. The equation for this reaction is.
Please check Write the balanced equation with the enthalpy component for the rusting process given that Hf for iron and oxygen is 0 and Hf for ferric oxide is -826 kJmol. The reactants are _____ and the product is _____ oxygen and iron iron oxide. Iron metal reacts with oxygen gas to form rust ironiiioxide.
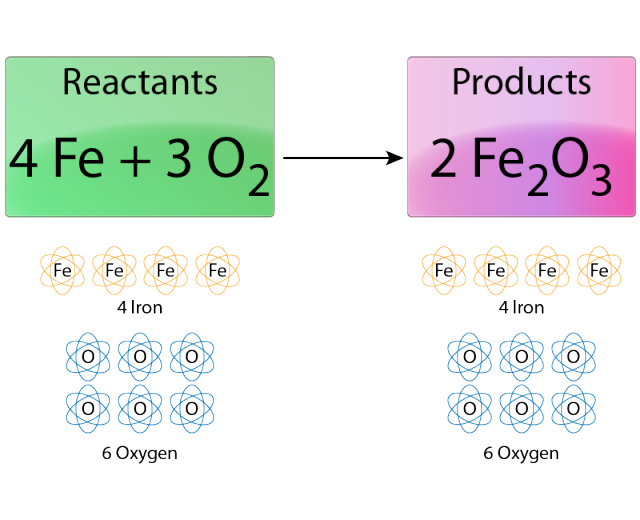
4Fe 3O2 2Fe2O3. Complete the balanced chemical equation in the data table. My experience is the iron would rust faster and I assume thats because iron is more reactive than copper so it will release electrons easier.
Just enter the correct number in decimal format rounded to the correct number of significant figures. 4 Fe s 3 O2 g -------- 2 Fe2O3 s You need to add the heat one of three. Note that this is about halfway between iron III hydroxide Fe OH 3 or ½ Fe 2 O 3 3H 2 O and anhydrous Fe 2 O 3.
The rusting of iron is characterized by the formation of a layer of a red. 4Fe 3O2 2Fe2O3. Two examples of combustion reactions are.
Water is also required for this reaction to occur but because the total amount of water does not change it is not included in the equation. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Metal oxygen metal oxide.



:max_bytes(150000):strip_icc()/BalanceEquations1-56a132765f9b58b7d0bcf535.png)