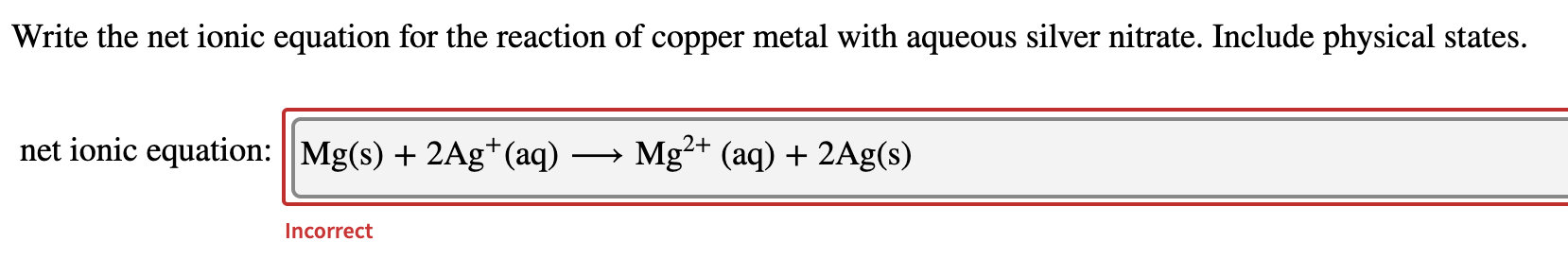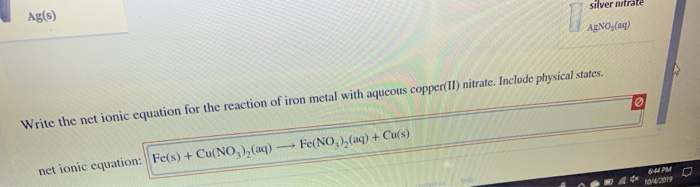Smart Copper And Silver Nitrate Net Ionic Equation
We review their content and use your feedback to keep the quality high.
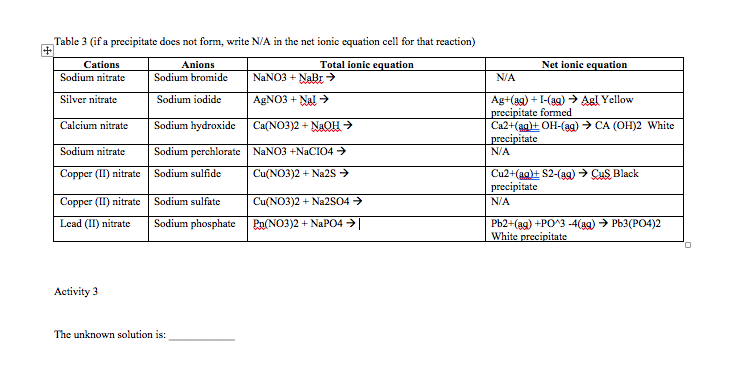
Copper and silver nitrate net ionic equation. The balanced equation for the reaction is. Starting with 150ml of 0250M silver nitrate AgNO 3 how many moles of AgNO 3 is that. Calcium sulfide leadII nitrate.
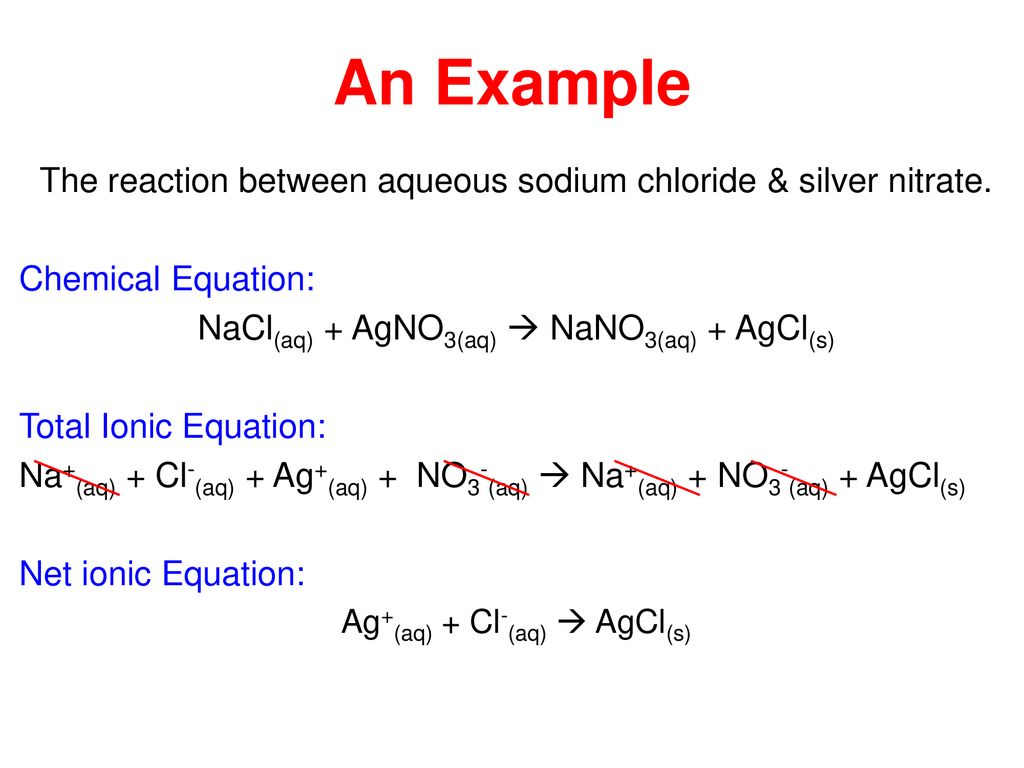
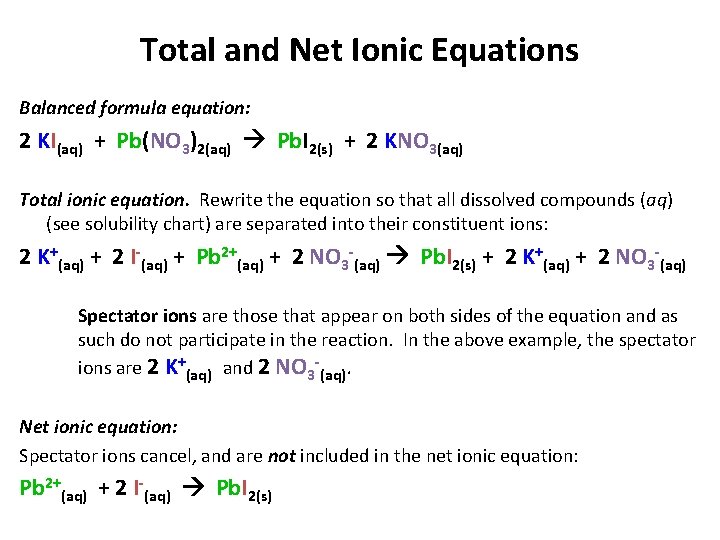
Ag aq Cl-aq à AgCls 2. The molecular equation is. Reaction may be described by the net ionic Equation 1115.
Experts are tested by Chegg as specialists in their subject area. When two solutions of ionic compounds are mixed a solid may form. 1 Potassium phosphate and calcium nitrate.
AgNO_3aqKClaq-AgClsKNO_3aq During this reaction a precipitate will form which is the silver chloride AgCl. Cu s 2AgNO 3 aq Cu NO 3 2 aq 2Ag s. Sodium carbonate potassium nitrate.
Cu s 2AgNO3aq 2Ag s Cu NO32aq The ionic equation includes all of the ions in the reactants and products. Molecular Y s PCI 2 Barium chloride and sodium sulfate. The silver in the silver nitrate.
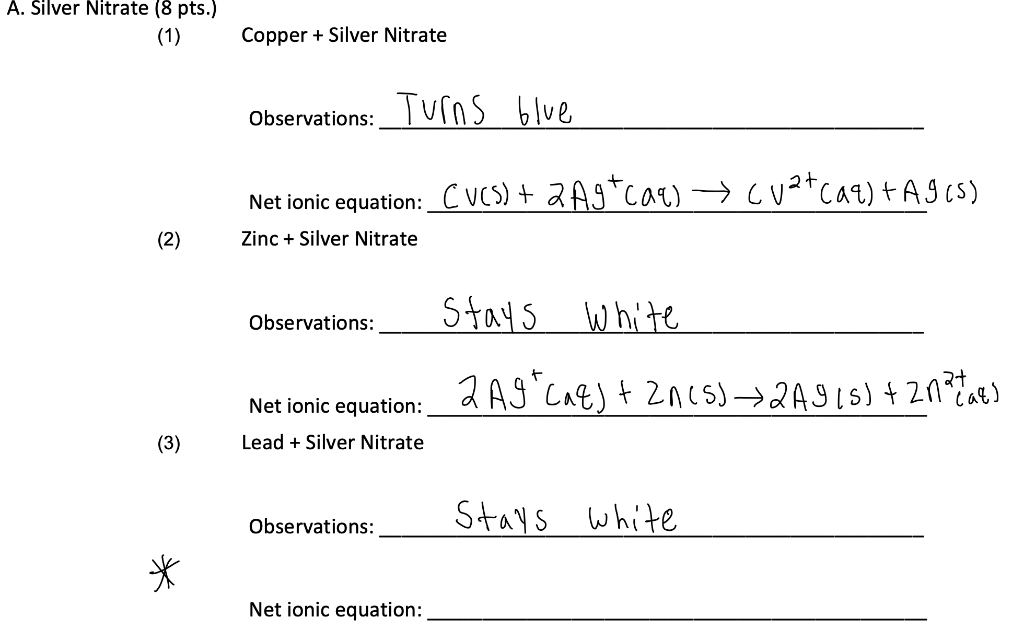
Total-ionic - 3 Sodium ana nitrate. A common type of displacement reaction takes place when a reactive metal reacts with the salt of a less reactive metal. Copper metal dissolves in a silver nitrate solution.