Recommendation Rusting Of Iron Nail Is Which Change
Is a nail rusting a physical or chemical change.
Rusting of iron nail is which change. The colour of the surface of the iron also changes. Rust is clearly a substance that is different from iron. Rusting of iron is a chemical change because a new substance called iron oxide is formed during this process.
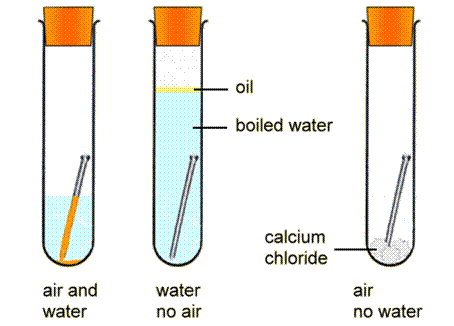
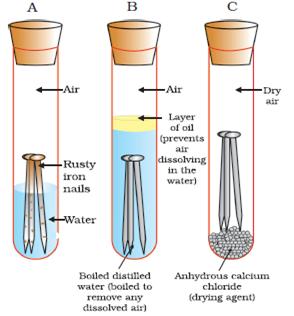
Iron is susceptible to rusting Rusting can only occur in the presence of moisture water and air Controlling humidity test tube C prevents rusting Galvanising using a more reactive metal such as zinc prevents the rusting of iron. Rusting of an iron nail is an example of a change of phase. Chemical Causes of Rusting The causes of corrosion require the presence of water and oxygen.
Allow the testtubes to rust for two weeks. The rusting of iron is a chemical change because it is two substances reacting together to make a new substance. Report an issue.
Iron Oxygen from environment Water Humidity Iron Oxide Rust. The evidence of chemical reaction in the rusting of an iron nail is a color change. Water and Carbon dioxide.
Rusting is a type of Chemical Change. The formation of a reddish brown flakes which loosely adheres to the iron is called rust. Chemical Reaction of Rusting is as follows.
Hence rusting of iron is a chemical change. After two weeks mesuare the change in the mass of each nail. I also think the color of the water will change.