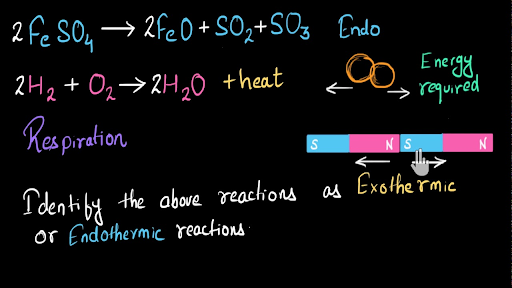Nice Endothermic Reaction Examples With Equations

In photosynthesis plants make the simple sugar glucose C 6 H 12 O 6 from carbon dioxide CO 2 and water H 2 O.
Endothermic reaction examples with equations. It makes it very easy for a user to balance their chemical equation afterwards. The reactions which occur by the absorption of heat energy either in the form of light or electricity are called endothermic reactions. C s O 2 g.
So all the decomposition reactions are endothermic reactions. In this reaction 43 kcal are needed to make the reaction occur. Energy in the form of heat.
The reaction equationNH4NO3 s water NH4 aq NO3 aqThis is an example of endothermic reaction because the temperature drops because heat energy is taken in by the reaction mixture. A few examples of the endothermic process are photosynthesis evaporating liquids melting ice dry ice alkanes cracking thermal decomposition ammonium chloride in water and much more. Characteristics equations and examples A endothermic reaction It i one that to take place mut aborb energy in the form of heat or radiation from it urrounding.
Converting frost to water vapor melting boiling and evaporation in general are endothermic processes. If the products contain more energy than the reactants heat is taken in or absorbed from the surroundings and the change is called an endothermic reaction endothermic energy transfer endothermic energy change of the system. They also release oxygen O 2 in the process.
Some examples of endothermic chemical reactions and processes are given. The reaction produces heat or endothermic reactions are lower on ferrous sulphate and examples of exothermic endothermic equations. Heat location is the quickest way to find if a reaction is endothermic or exothermic.
Energy is released as heat electricity light or sound. This implies that the energy needed to initiate the reaction is less than the energy that is subsequently released. 6CO2g 6H2O l energy C6H12O6s 6O2g 6 CO 2 g 6 H 2 O l energy C 6 H 12 O 6 s 6 O 2 g Photosynthesis is an endothermic reaction.