Impressive Ammonia And Hcl Equation

Ammonia reacts with oxygen gas when heat is supplied and ammonia is oxidized as a result.
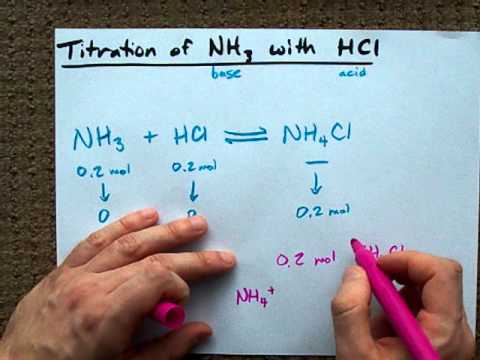
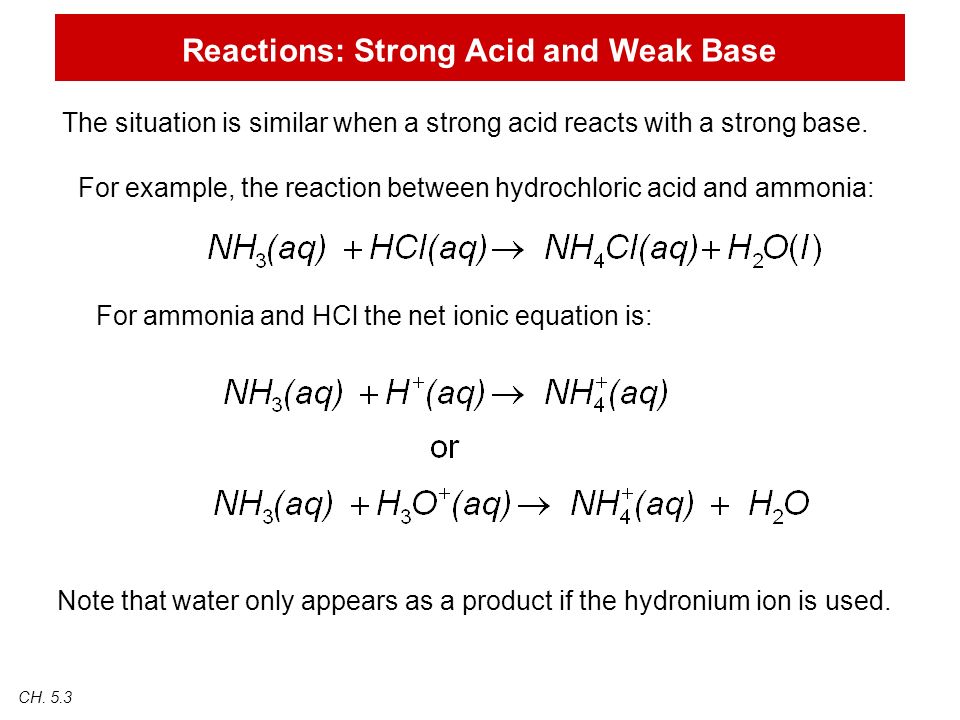
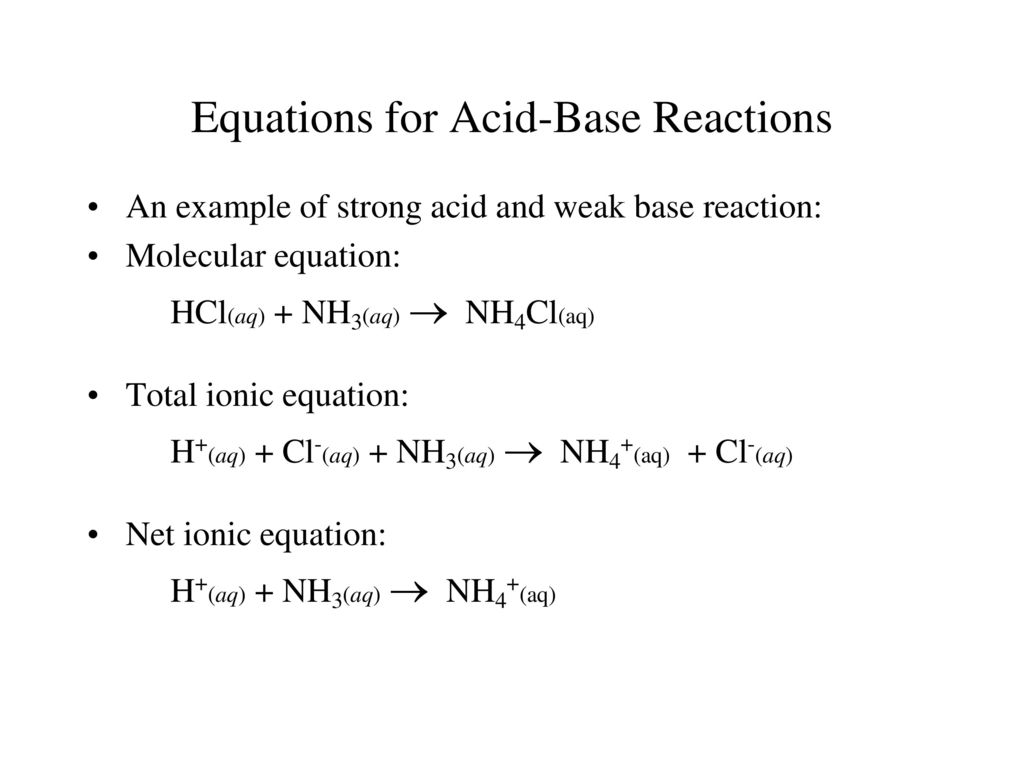
Ammonia and hcl equation. NH 3 aq HCl aq NH 4 aq Cl aq Hydrochloric acid and the chlorine ion are one conjugate acid-base pair and the ammonium ion and ammonia are the other. MATERIAL SAFETY DATA SHEET MSDS AMMONIA. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
You could write this equation in two ways. Additionally is HCl a strong acid. By Staff Writer Last Updated March 26 2020 The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
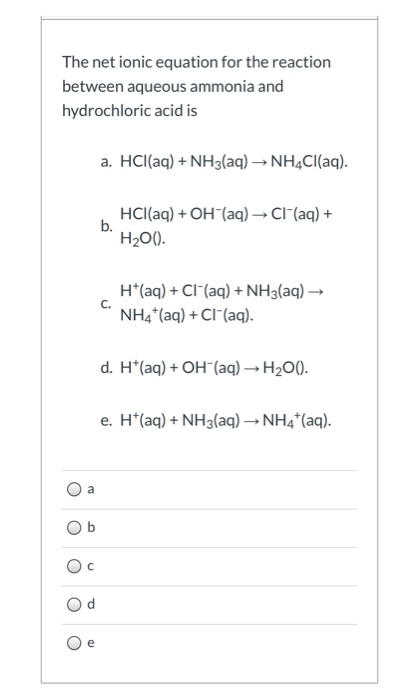
NH3aq HClaq NH4Claq NH4OHaq HClaq NH4Claq H2Ol. This is how a buffer maintains a near constant pH. HCl aq H 2 O l H 3 O aq Cl aq Using the Brønsted-Lowry theory the reaction of ammonia and hydrochloric acid in water is represented by the following equation.
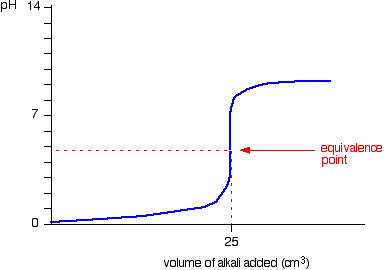
Ammonia solution reacts with hydrochloric acid a solution of hydrogen chloride in water in a similar way. NH3 H NH4. A reminder about the ammonia reactions.
If you mix together a solution of ethanoic acid and a solution of ammonia you will get a colorless solution of ammonium ethanoate. The reaction of hydrochloric acid HCl with ammonia NH3 is described by the equation. Also this reaction occurs with catalyst and without catalyst under different conditions and you will learn everything.
NH 3 HCl NH 4 Cl. Click to see full answer. Every buffer is made up of a conjugate acid-base pair.