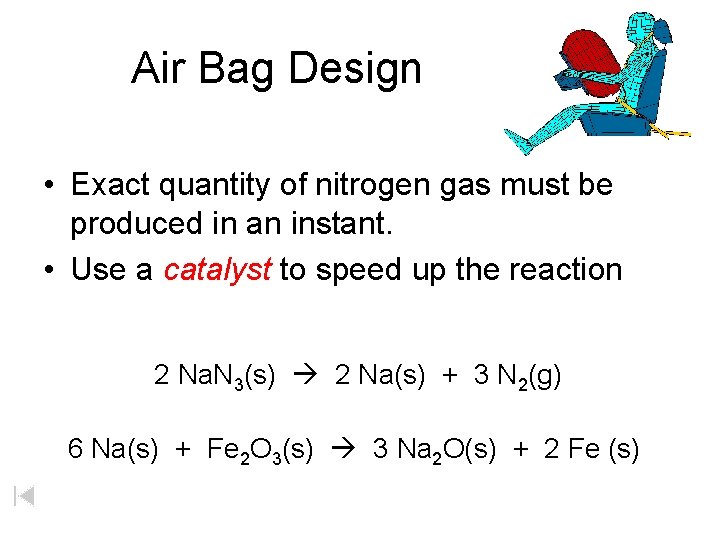Brilliant Airbag Reaction Equation

The human is traveling at 156 ms inside a car that just stopped instantly.
Airbag reaction equation. And their chemical formulas involved in airbag deployment based on the. 2Sort the chemicals in two ways. The purpose of automobile airbags is to protect a driver or passenger when they are involved in an automobile accident.
2NaN3s 3N2g 2Nas 2 N a N 3 s 3 N 2 g 2 N a s This reaction is very rapid generating gaseous nitrogen that can deploy and fully inflate a typical airbag in a fraction of a second 00301 s. 2 NaN3 2 Na 3 N2. Write a balanced equation for the net gas-generating reactions the combination of the first and second reactions.
The reaction in an air bag Chemical reactions occur in our lives everyday often chemical reactions are intentionally created in order to solve a solution or even save a life. K20 Na20 SiO2 alkaline silicate glass. The things you have to think about are amount of substances in reaction time of reaction temp.
Carbon dioxide water and sodium acetate are the products of. If heated though it will fall apart. 338 moles of sodium azide must be packed into the air bag module for the air bag to inflate PVnRT P12 atm v70L R0821 L atm K-1 mol-1 T30273.
Outline the steps necessary to determine the number of moles and mass of H 2. Under normal circumstances this molecule is quite stable. 1 Substituting in the values above we have 894 ms2 - 000 ms2 2a0300 m a 133x104 ms2.
10 Na 2 KNO3 K2O 5 Na2O N2. This is important because itll determine. NaN3 Na N2 g Na Second Reaction.