Simple Naoh And Hcl Balanced Equation
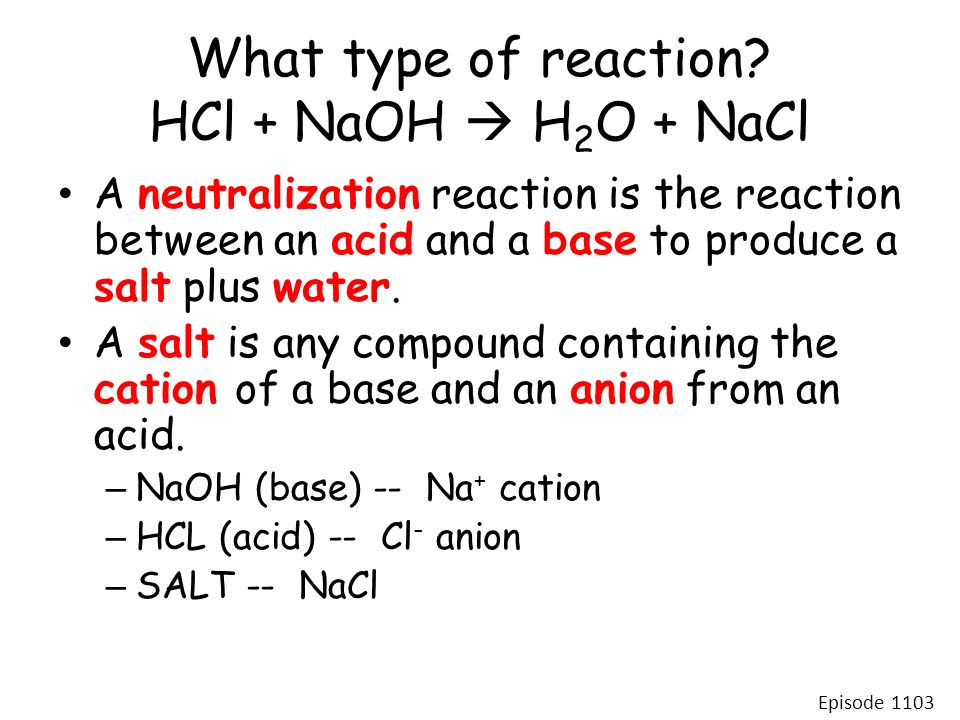
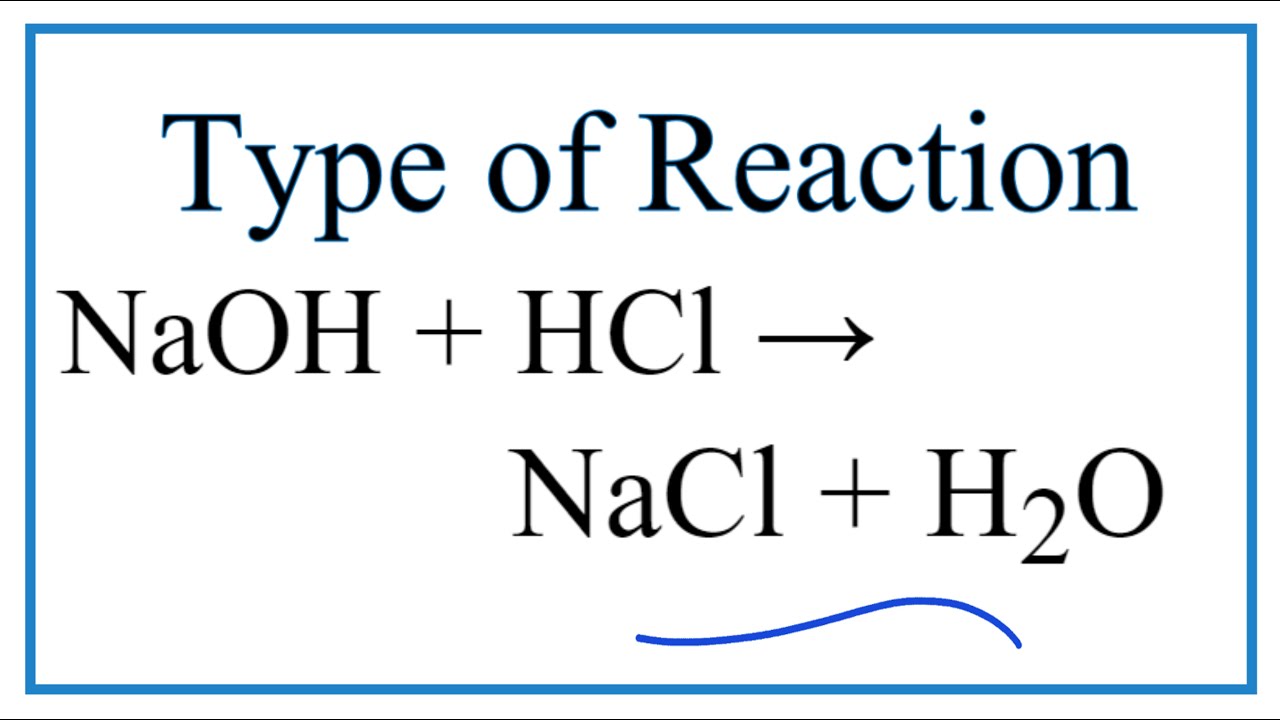
In this equation they react to form the products NaCL sodium chloride or salt and H2O water.
Naoh and hcl balanced equation. 125mL 1 M ML 010054L 00054mol 2 00054mol NaOH X 1 mol HCl 00054mol 1. Then a chemical equation can be written HCl NaOH NaCl H2O NaOH is a reactant in the equation. Fe Au Co Br C O N F.
HCl NaOH Na2B4O710H2O H2O NaCl H3BO3. K 4 FeCN 6 H 2 SO 4 H 2 O K 2 SO 4 FeSO 4 NH 4. Examples of complete chemical equations to balance.
For instance in the equation HCl NaOH NaCL H2O the HCl hydrochloric acid a strong acid and NaOH sodium hydroxide a strong base are the reactants. KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2. This means that we will split them apart in the net ionic equation.
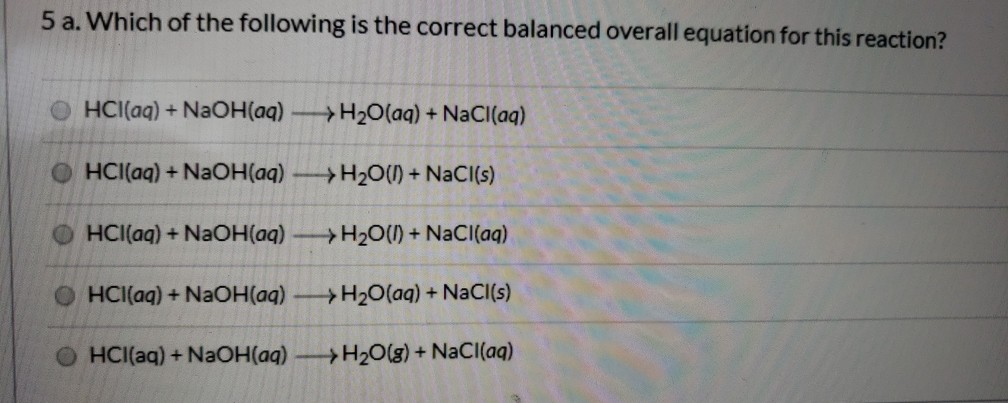
Search by reactants NaOH HCl and by products NaCl H 2O 1 HCl NaOH H2O NaCl. The balanced chemical equation representing the neutralization of hydrochloric acid with sodium hydroxide is. HCl NaOH KOH H2O NaCl KCl.
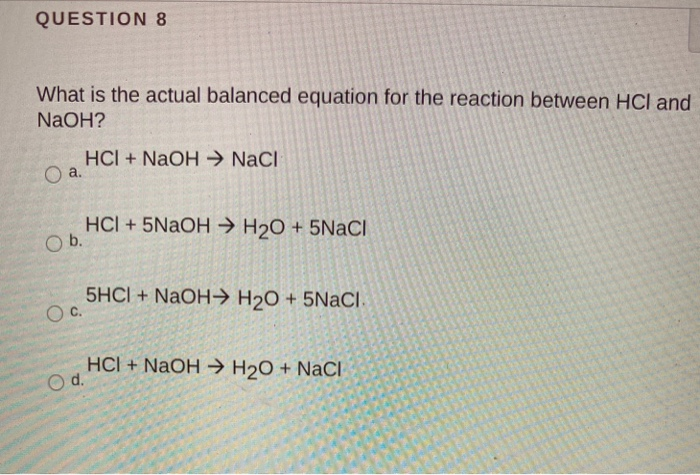
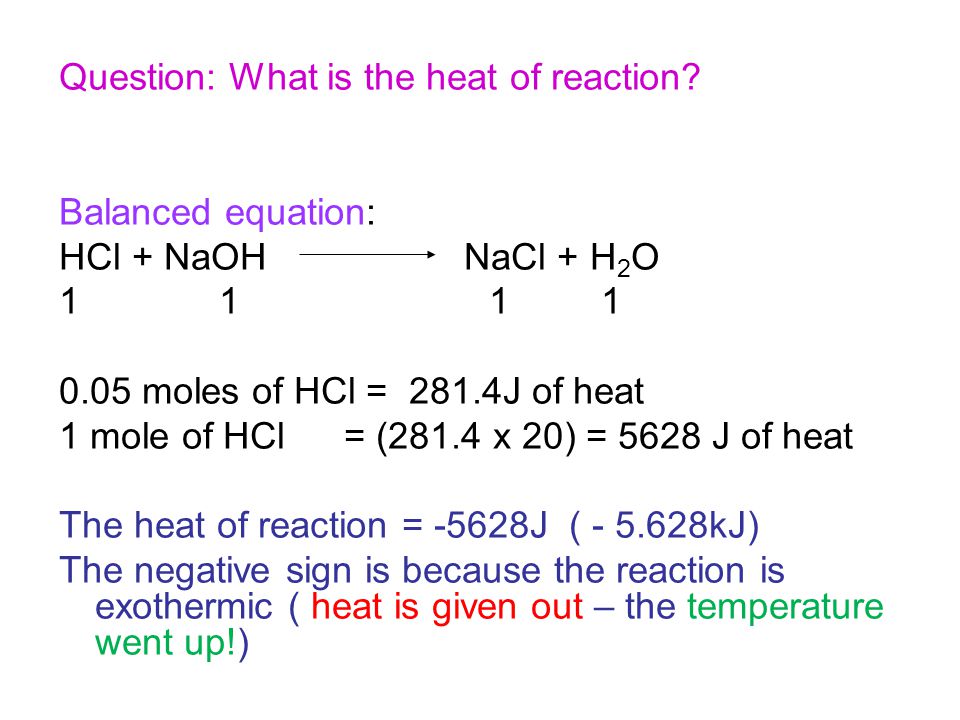
First we balance the molecula. What is the limiting reactant in this chemical equationIn the neutralization of 10 M HCl and 10 M NaOH NaOH aq HCl aq - NaCl aq H2O. HCl aq NaOH aq NaCl aq H2O l heat Calculate the number of moles of base you add to determine the molar heat of neutralization expressed using the equation ΔH Q n where n is the number of moles.
It is not possible to have a a balanced chemical equation for NaOH. Phenolphthalein indicator pH range 8. How to Balance the Net Ionic Equation for HClO 4 NaOH The reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base.