Brilliant Butane Burned In Oxygen Equation

Hence moles of oxygen are required to burn 1 mol of butane.
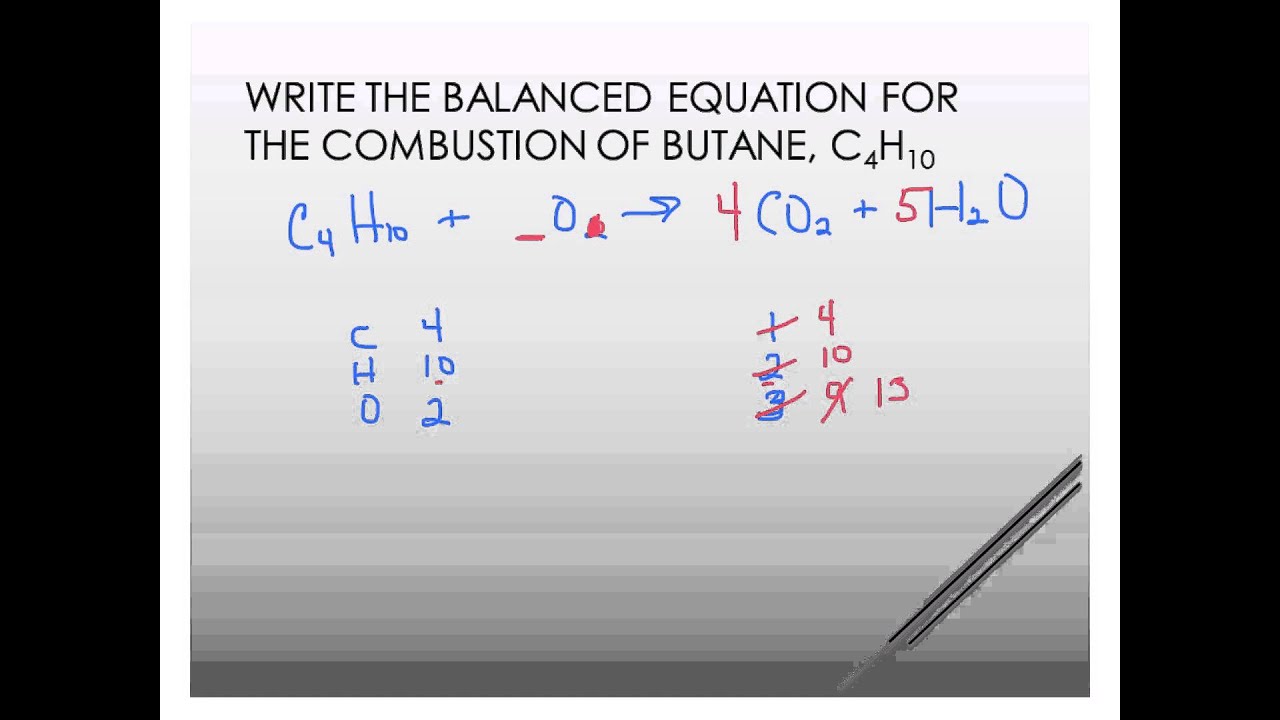
Butane burned in oxygen equation. The balanced equation for the combustion of butane C4H10 is 2 C4H10g 13 O2g 8 CO2g 10 H2Og. Carbon monoxide can react with oxygen to form carbon dioxide. Which statement is correct.
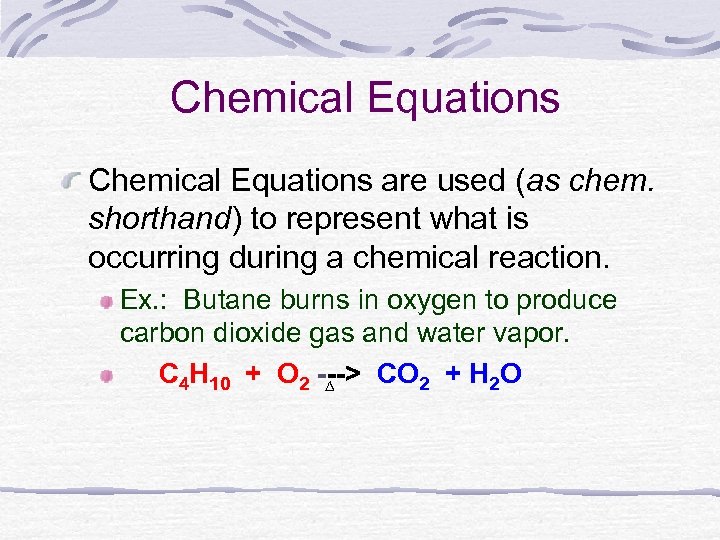
The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water. Is butane burning in a butane lighter a chemical reaction. An equation for the incomplete combustion of butane in oxygen is.
2C4H10g 13O2g 8CO2g 10H2O g. Butane is a gas at room temperature and pressure. How many moles of CO2 form when 580 g of butane C4H10 burn in oxygen.
C4H10 g 65 O2 g --------- 4 CO2 g 5 H2O g 20 cm3 of butane are completely burned in 020 dm3 of oxygen. E Ammonium nitrate Nitrogen Carbon dioxide water. The equation below represents the complete combustion of butane.
How many moles of water vapour are formed when 10L of butane gas C4H10is burned in oxygen at STP. Click card to see definition 22 moles Click again to see term. The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water.
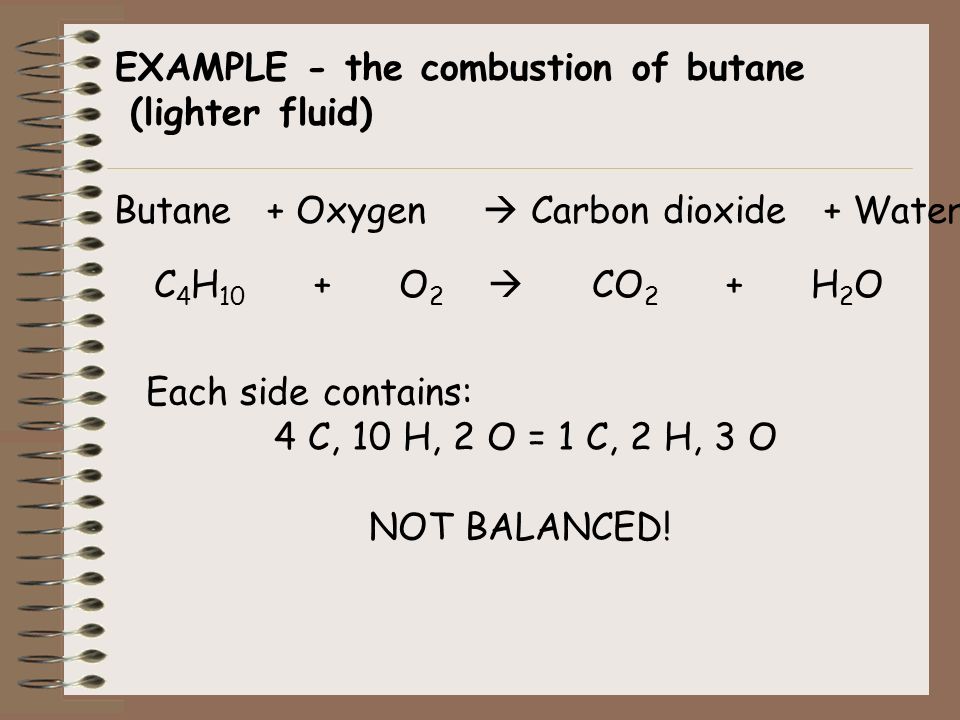
Complete combustion does NOT give carbon monoxide or sootCheck me out. This is the basic UNBALANCED equation. Chapter 2 Problem 48P is solved.