Exemplary Balanced Equation Combustion Of Propane

C₃H₈ 5O₂ 3CO₂ 4H₂O 32 views Answer requested by.
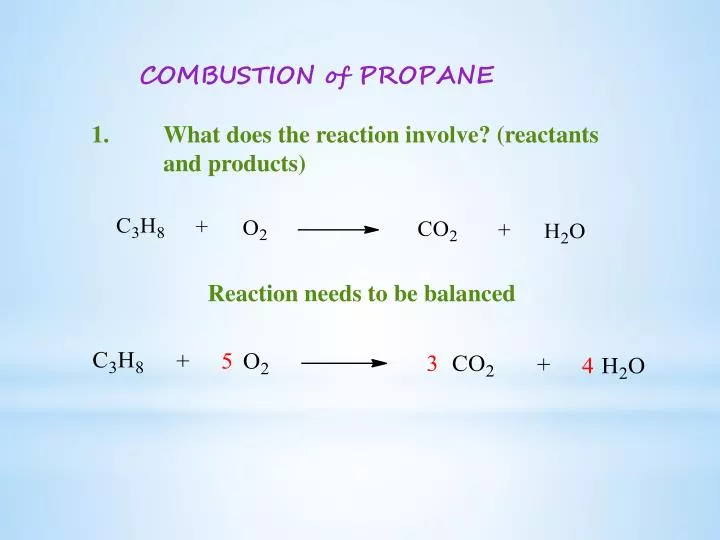
Balanced equation combustion of propane. The first one is a combustion reaction. Youre dealing with the combustion of propane. Propane oxygen carbon dioxide water.
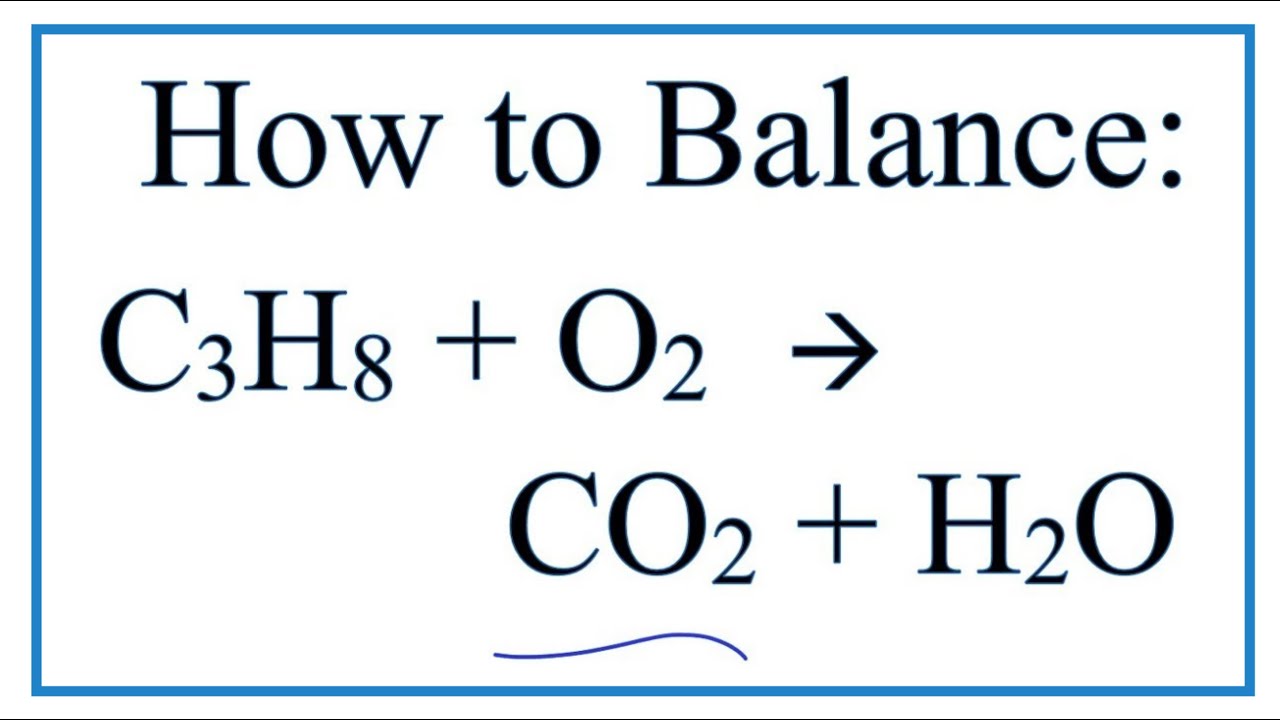
Complete combustion does NOT give carbon monoxide or sootCheck me out. By signing up youll get thousands of step-by-step solutions to your. Chemistry Write a balanced equation for the combustion of gaseous propane C3H8 a minority component of natural gas in which it combines with gaseous oxygen to form gaseous carbon dioxide and gaseous water.
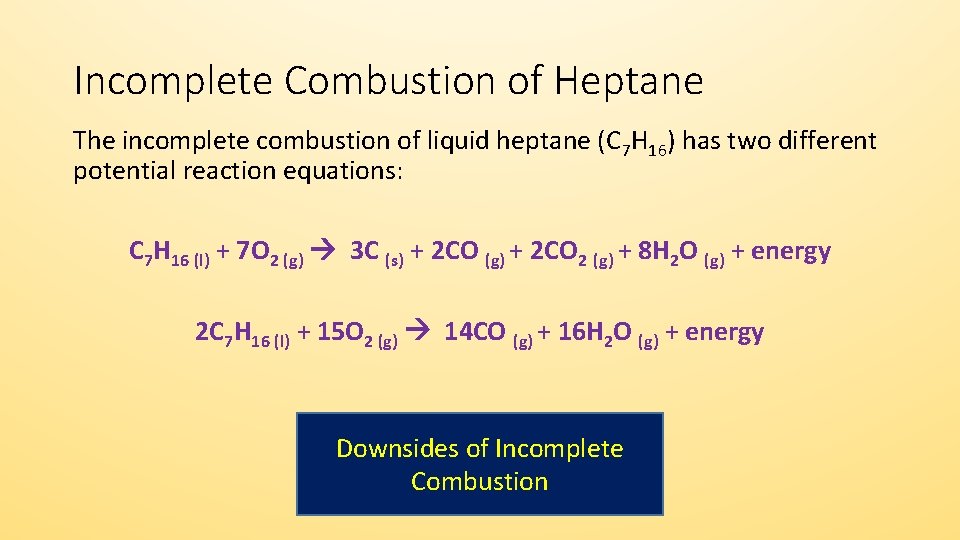
Incomplete combustion produces carbon monoxide which is a poisonous gas. C 3 H 8 3 O 2 2CO C 4H 2 O C 3 H 8 25 O 2 CO 2C 4H 2 O b Incomplete Combustion Equation -. Complete combustion of LPG propane yields about 25 MJlitre or 49 MJkg of heat.
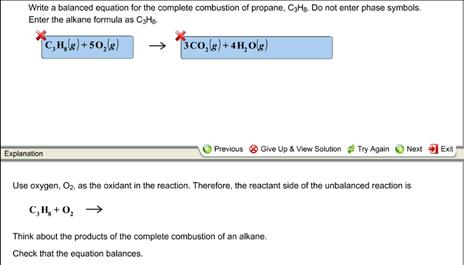
The balanced chemical equation looks like this C3H 8 5O2 3CO2 4H 2O Now the tool you have at your disposal when doing limiting reagent problems or any stoichiometry problem is the mole ratio. Fuel O2 -CO2 H2O The coefficients of the balanced equation will change depending on the fuel. With the states of matter describing the complete combustion of propane gas C3H8.
The incomplete combustion of propane C 3 H 8 produces two possible balanced equations. The complete combustion of propane produces carbon dioxide gas and water vapor. The balanced equation shows the complete combustion of propane in oxygen or in air to form carbon dioxide and water ie.
The balanced chemical equation for the combustion of propane a common heating fuel is mathrmC_3 mathrmH_85 mathrmO_2 longrightarrow 3 mathr. The propane equation for complete combustion of propane involves propane and oxygen as fuel input and carbon dioxide water heat and possible carbon monoxide as the outputs. Write a balanced equation for the combustion of gaseous propane C3H8 a minority component of natural gas in which it combines with gaseous oxygen to form gaseous carbon dioxide and gaseous water.